 |
 |
 |
| |
Maraviroc Study in Dual/Mixed Tropic Patients
|
| |
| |
Reported by Jules Levin
XVI Intl AIDS Conference, Toronto, Aug 17, 2006
"Safety and Efficacy of MARAVIROC, a Novel CCR5 Antagonist, When Used in Combination with Optimized Background Therapy for the Treatment of Antiretroviral-Experienced Subjects Infected with Dual/Mixed-Tropic HIV-1: 24-Week Results of a Phase 2b Exploratory Trial"
H Mayer1, E van der Ryst2, M Saag3, B Clotet4,
G Fatkenheuer5, N Clumeck6, K Turner2, and JM Goodrich1
Pfizer Global Research and Development, 1New London, USA, and 2Sandwich, UK, 3University of Alabama at Birmingham, USA, 4University Hospital Germans
Trias i Pujol, Barcelona, Spain, 5University of Cologne, Cologne, Germany,
6St Pierre University Hospital, Brussels, Belgium
Author's Summary.
Over 24 weeks, in treatment-experienced patients with D/M-tropic HIV-1 and advanced disease:
MARAVIROC + OBT was generally well tolerated
- No cases of hepatotoxicity, lymphoma or adenocarcinoma
MARAVIROC + OBT did not demonstrate superior reductions in HIV-1 RNA compared with placebo + OBT (but viral load did not decrease)
MARAVIROC + OBT was associated with greater increases
in CD4 cell count than placebo + OBT
Patients receiving MARAVIROC + OBT were more likely to fail with X4-tropic HIV-1 than those receiving placebo + OBT
- However, patients treated with MARAVIROC and X4-tropic virus at treatment failure had increases in CD4 cell count consistent with the overall MARAVIROC-treated population
Howard Mayer reported the results of Study 1029 at the Late Breaker Oral Session on Thursday Aug 17,2006. A brief explanation is in order to help put these study results in context.
Mean CD4 counts and viral loads were not negatively affected in this study, in fact mean CD4 counts increased for patients on Maraviroc, by patients with dual/mixed tropic HIV taking Maraviroc. There were no apparent safety issues related to Maraviroc. The study suggests it may be safe for dual/mixed tropic patients to take Maraviroc.
There did not appear in this study to be harm done to viral load or CD4 count, in fact CD4 count increased for patients on Maraviroc, by patients with dual/mixed tropic HIV taking Maraviroc. There were no apparent safety issues related to Maraviroc. The study suggests it may be safe for dual/mixed tropic patients to take Maraviroc.
When HIV enters a CD4 cell where it reproduces itself, it must attach and bind to the cell first. As part of the process of binding and entering the cell HIV attaches itself to 2 co-receptors on the cell surface called CCR5 and CXCR4. Maraviroc is a CCR5 antagonist, meaning it prevents HIV from binding to the CCR5 co-receptor and thus prevents HIV from entering the cell and reproducing itself. For the most part, patients in early HIV disease use CCR5 to attach and enter, but when patients have advanced disease they often switch to using CXCR4 or they may use both. There is a test you can use (Trofile, from Monogram Biosciences, which also makes resistance tests) which can tell you whether you use CCR5 or CXCR4 to bind. It is however uncertain if it is the advanced disease that tells HIV to use CXCR4 to bind or if by switching to CXCR4 to bind this causes HIV to advance. If a person's HIV uses CCR5 to attach they are called CCR5 tropic. If the patient's HIV uses CXCR4 to bind they are called CXCR4 tropic. And some patients may have some HIV in their blood that uses CCR5 to attach and some HIV in their blood at the same time that uses CXCR4 to attach, or perhaps they have HIV that uses both CCR5 and CXCR4 to attach. These patients are called mixed/dual tropic.
I hope this explanation was not confusing. So, this study was conducted to see if dual/mixed tropic patients would be harmed if they take Maraviroc.
Background
-- Maraviroc is a selective, reversible CCR5 (R5) antagonist
-- It is active in vitro (in the test tube) versus R5-tropic HIV-1, MDR HIV-1 but not X4-tropic or R5X4-tropic HIV-1
-- It has Cross-clade activity (~ 2 nM antiviral IC90)
In Phase 1/2a clinical trials:
-- Generally well tolerated in healthy volunteers and patients
-- Maximum HIV-1 RNA reductions of >1 log10 copies/mL in all patients with R5 tropic HIV-1 treated with MVC monotherapy at ≥100 mg BID
MARAVIROC Phase 2b/3 Program to Assess Safety and Efficacy in Patients with R5-tropic HIV-1
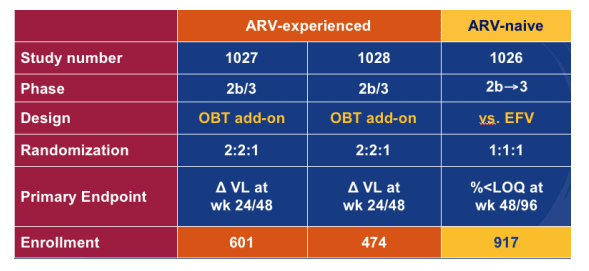
There are 3 phase 3 studies ongoing, one in treatment-naive patients and 2 in treatment-experienced patients listed in this table.
Rationale for Studying MARAVIROC in Patients with Dual/Mixed (D/M)-Tropic Infections
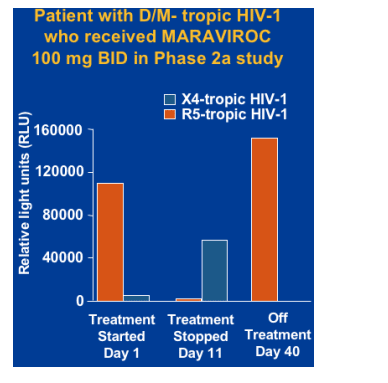
Theoretical risk of outgrowth of X4-tropic HIV-1 when a patient with D/M-tropic HIV-1 is treated with a CCR5 antagonist.
X4-tropic HIV-1 has been associated with more rapid CD4 cell depletion and progression to AIDS.
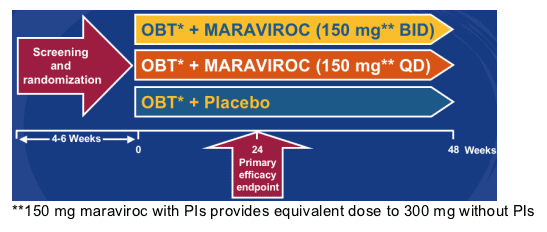
Phase 2b Pilot Study Evaluating the Safety of MARAVIROC in Patients with Non-R5 HIV-1
Patients were randomized to receive Maraviroc 150 mg bid plus optimized background therapy (OBT), or Maraviroc 150 mg qd (once daily) plus OBT, or just OBT (Maraviroc placebo).
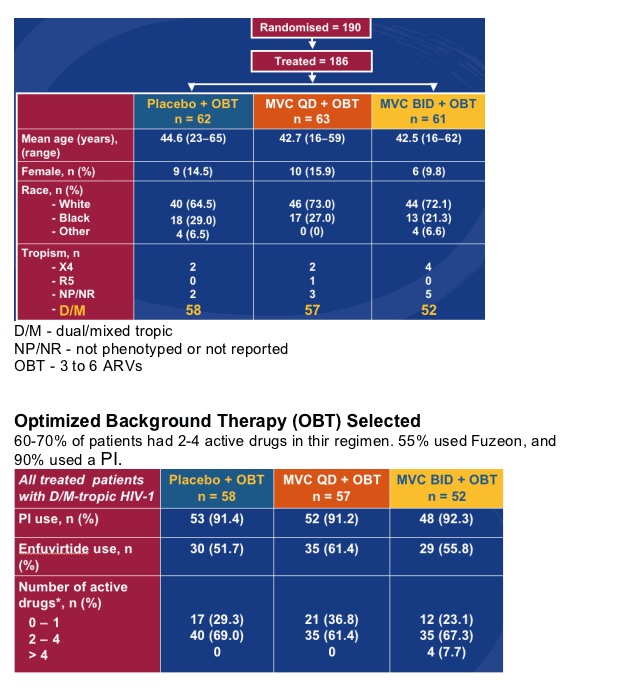
-- Randomized, double-blind, placebo-controlled study
Selection criteria:
- D/M-tropic
- Antiretroviral experienced and/or multi-class resistance
- At least one active drug in OBT
STUDY POPULATION
There were 58 dual/mixed tropic patients in the placebo group, 57 in the MVC qd+OBT group, and 52 in the MVC bid+OBT group. 20-30% of the patients were Black and 64-73% were White. 10-15% were women.
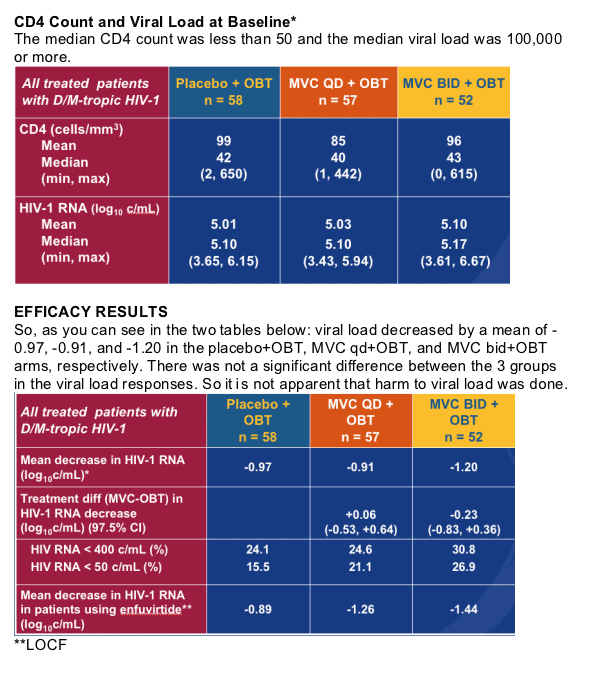
Changes in CD4 Cell Count
Patients with D/M-tropic HIV-1 at Screening
There were some patients in the study who had dual/mixed tropic HIV ar=t the beginning of this study had only X4 tropic HIV detected at time of virologic failure. But encouragingly, their CD4 counts increased, as you can see in the table. So, the drug does not appear to harm them.
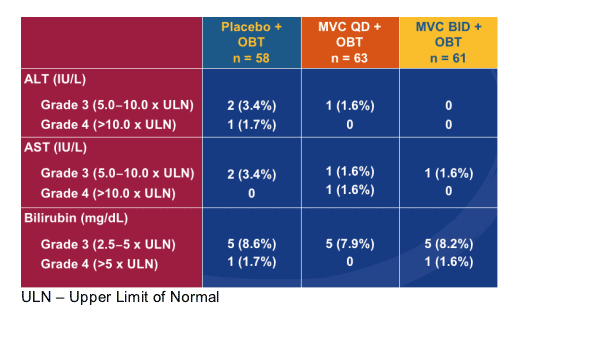
Summary of Clinical and Laboratory Adverse Events
In general, MARAVIROC was well tolerated
-- AEs, including those judged to be severe or life threatening (Grade 3/4), SAEs and discontinuations due to AEs were evenly balanced between treatment groups
-- No cases of lymphoma or adenocarcinoma reported
--Thirteen category C events occurred:
MARAVIROC QD (7), MARAVIROC BID (3), Placebo (3)
There were 7 deaths in patients on study drug and none were considered drug related
MARAVIROC QD (2), MARAVIROC BID (2), Placebo (3)
Grade 3/4 LFT Abnormalities
(Without Regard to Baseline)
|
| |
|
 |
 |
|
|