 |
 |
 |
| |
EFV +TDF/FTC or Combivir 96 Wks Study 934: limb fat/renal/resistance/safety/viral efficacy
|
| |
| |
Efficacy and Safety of Tenofovir DF (TDF), Emtricitabine (FTC) and Efavirenz (EFV) Compared to Fixed Dose Zidovudine/ Lamivudine (CBV) and EFV Through 96 Weeks in Antiretroviral Treatment-Na´ve Patients
Study 934
Reported by Jules Levin
Poster Number, TUPE0064
XVI International AIDS Conference, August, 2006, Toronto, Canada
JE Gallant1, AL Pozniak2, E DeJesus3, JR Arribas4, R Campo5, S-S Chen6,
D McColl6, J Enejosa6, and AK Cheng6 for the Study 934 Team
1Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Chelsea and Westminster
Hospital, London, UK; 3Orlando Immunology Center, Altamonte Springs, FL, USA; 4University Hospital
La Paz, Madrid, Spain; 5University of Miami, Miami, FL, USA; 6Gilead Sciences, Inc., Foster City, CA, USA
This is a large phase III trial comparing once daily TDF, FTC and efavirenz (EFV) to twice daily CBV (AZT+3TC) and once daily EFV:
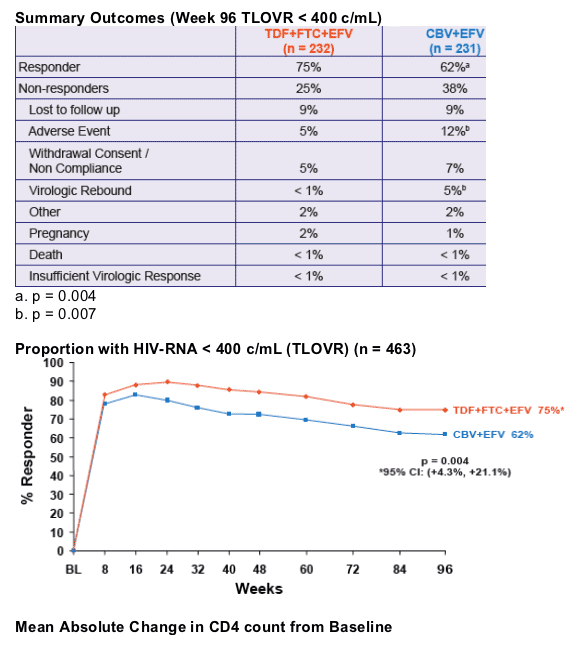
- At Week 48, the proportion of patients reaching primary endpoint of HIV RNA < 400 c/mL using the FDA TLOVR algorithm was significantly higher in the TDF+FTC+EFV arm1
- This presentation provides the results of Week 96 data analysis
1. Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine and efavirenz vs. zidovudine, lamivudine and efavirenz for HIV.
N Engl J Med 2006; 354:251-60.
Author Conclusions
- The TDF+FTC+EFV arm was associated with
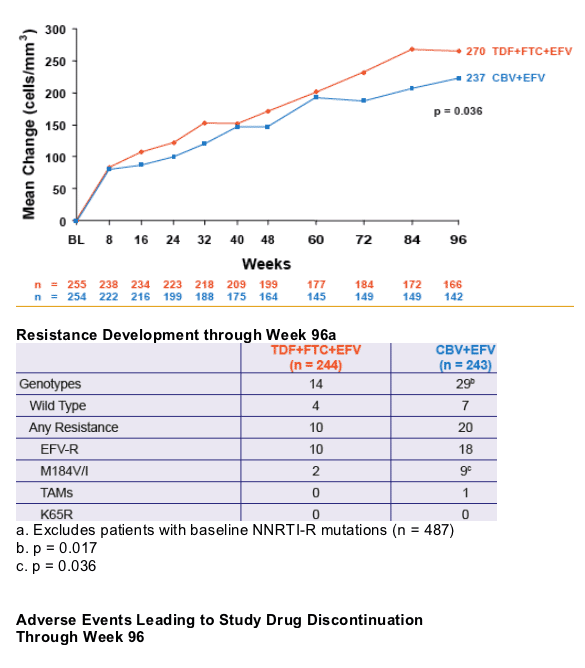
significantly greater virologic suppression to HIV RNA < 400 c/mL and a greater increase in CD4 cell count
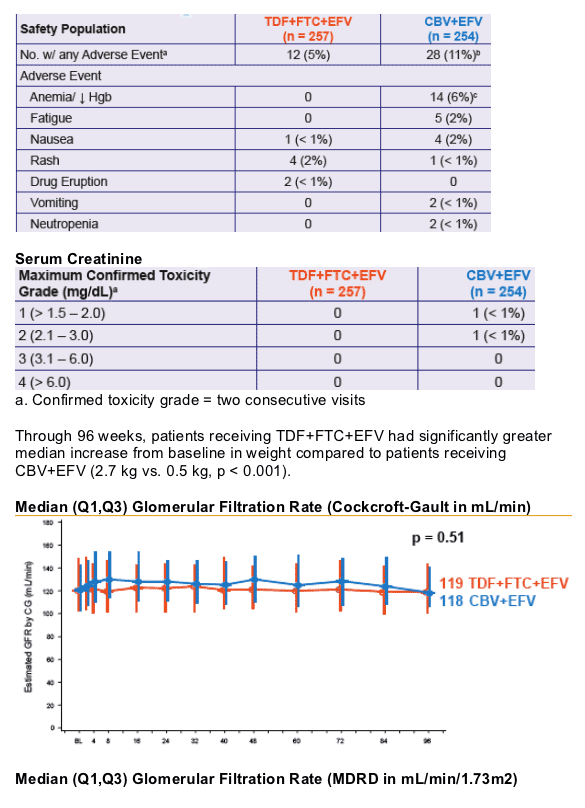
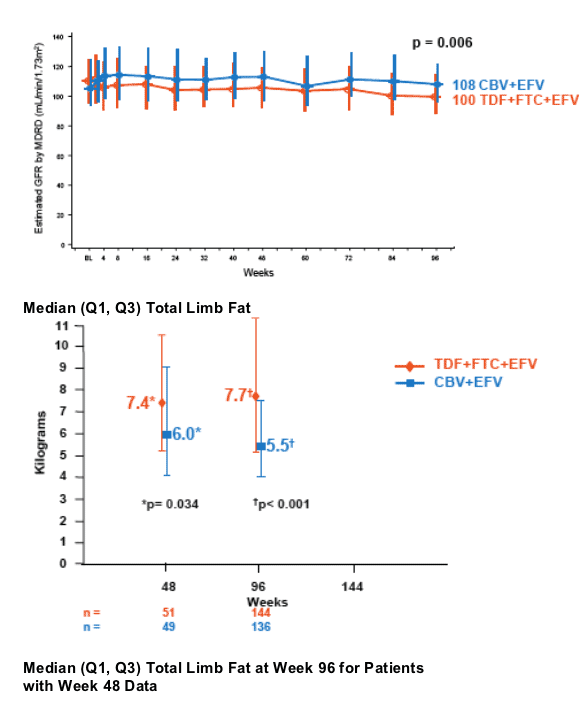
- Renal function remained stable through 96 weeks. No patient discontinued due to renal adverse events
- Significantly less M184V/I was seen in the TDF+FTC+EFV arm
- No emergence of K65R mutation was demonstrated
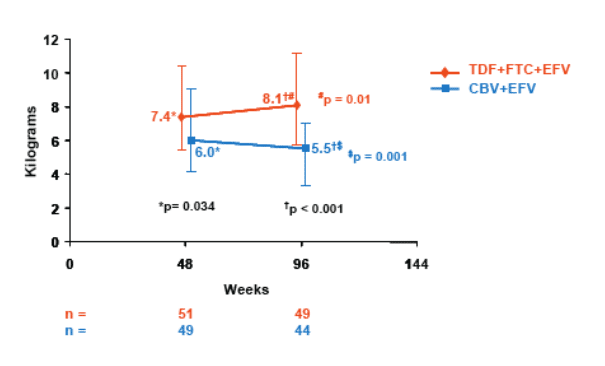
- Significantly higher limb fat was seen in TDF+FTC+EFV arm compared to CBV+EFV arm
- In a subset of patients with Week 48 and 96 data, a significant median decrease in limb fat was seen in the CBV+EFV arm and a significant increase in the TDF+FTC+EFV arm
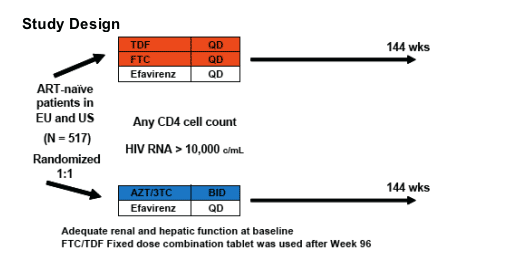
Statistical Analysis
- Non-inferiority trial with 48 week primary endpoint
- Effi cacy endpoint: Time to Loss of Virologic Response (TLOVR)
- Similar to ITT Missing = Failure, Switch = Failure (switching EFV to NVP due to CNS toxicity was not considered failure)
- Requires confi rmation for success
- FDA-required endpoint for presentation in U.S. Prescribing Information of newly approved antiretrovirals
- Week 96 Efficacy Patients:
- Patients with baseline NNRTI-R mutations and patients who completed the Week 48 study with HIV RNA below limit of quantification but did not consent to participate in the study extension from Weeks 48 - 96 were excluded from
the analysis
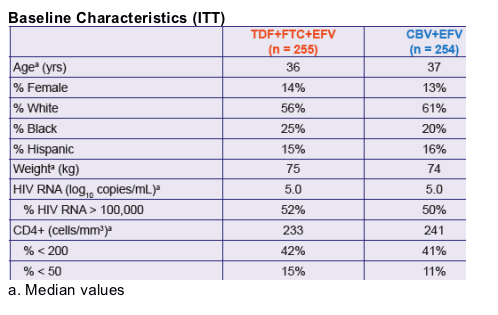
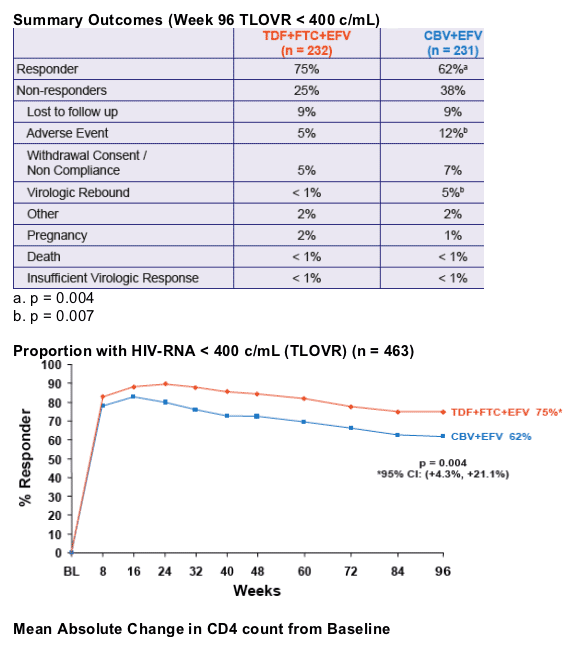
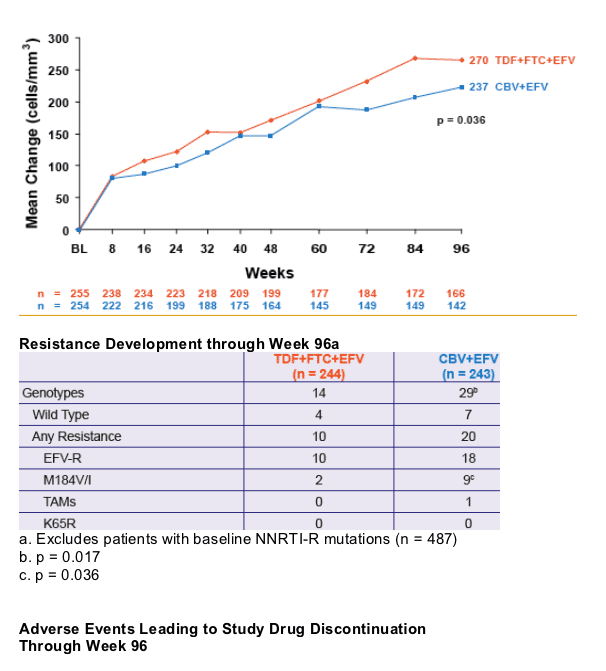
|
| |
|
 |
 |
|
|