 |
 |
 |
| |
Potent Antiretroviral Effect of MK-0518, a Novel HIV-1 Integrase Inhibitor, as part of Combination ART in Treatment -Naive HIV-1 infected Patients
|
| |
| |
Reported by Jules Levin
Authors: M. Markowitz1, B.-Y. Nguyen2, E. Gotuzzo3, F. Mendo4, W. Ratanasuwan5, C. Kovacs6, J. Zhao2, L. Gilde2, R. Isaacs2, H. Teppler2, and the Protocol 004 Part II Study Team
1Aaron Diamond AIDS Res. Ctr. New York, USA; 2Merck Res. Labs, West Point, USA; 3Hosp. Nac. Cayetano Heredia, Lima, Peru; 4Hosp. Nac. Edgardo Rebagliati, Lima, Peru; 6Maple Leaf Med. Ctr, Toronto, Canada; 5Siriraj Hosp., Bangkok, Thailand
Marty Markowitz reported the study results today at the World AIDS Conference. In this phase II study of the new Merck integrase inhibitor MK-0518 in treatment-naive patients, the drug was safe and very potent, and appears tolerable. The drug is taken twice a day and is on a fast track for FDA approval for treatment-experienced patients, and will be very useful for patients with extensive drug resistance who need it to compose a new regimen. Since this drug is from a new class of drugs patients should be fully sensitive to it. Merck announced this morning a worldwide Expanded Access Program, so anyone who needs the drug now can get access before its officially approved by the FDA. The current study reviewed here is in treatment-naive patients so this drug is being developed for firstline therapy and for salvage therapy as well.
Author Conclusions:
MK-0518 is a promising new strand transfer inhibitor of HIV integrase with potent and durable antiretroviral effect.
In treatment naive patients with HIV RNA ≥ 5000 copies/ml and CD4 ≥
100/mm3, MK-0518 at all doses studied for 24 weeks:
-- had potent antiretroviral activity
- 85-95% with HIV RNA < 50 copies/mL
- achieved viral suppression faster than EFV
-- was generally well tolerated
MK-0518: Strand Transfer Inhibitor of HIV Integrase
- HIV integrase inhibition: a new mechanism of action
- MK-0518: potent in vitro activity
- IC95 = 33 nM ± 23 nM in 50% human serum
- Preclinical evaluation favorable
- Metabolism primarily via glucuronidation (UGT1A1)
- Not a potent inhibitor or inducer of CYP3A4
- Does not require "ritonavir boosting"
- Phase I and drug interaction data support:
Dosing 100 - 800 mg po bid without regard to food
- At 100mg b.i.d, mean C12hr > IC95
No dose adjustment when used with other ARTs
STUDY DESIGN
8 patients each received MK-0518 at 600, 400, 200, or 100 mg bid or placebo for 10 days monotherapy. Then 30 patients in addition each received one of the MK-0518 doses plus TFV+3TC, or efavirenz plus TFV+3TC, for a total of 38 patients in each arm.
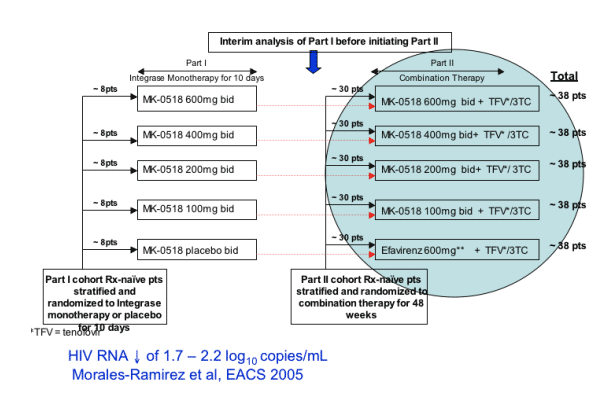
Protocol 004: Part II Design
Part I patients continued at same dose in Part II (pbo_efv)
150 additional patients randomized for Part II
Key inclusion criteria
-- Susceptible to EFV, 3TC , TFV (by genotype)
--No prior ART (<7 days OK)
-- HIV RNA ≥ 5000 copies/mL
baseline stratification for HIV RNA ≦ or > 50,000 copies/mL
-- CD4 ≥ 100 cells/mm3
Endpoints
-- HIV RNA and CD4 counts, Adverse experiences
Hypotheses: MK-0518 + TFV/3TC
-- will be generally safe and well tolerated
-- will have similar antiretroviral activity vs efavirenz + TFV/3TC
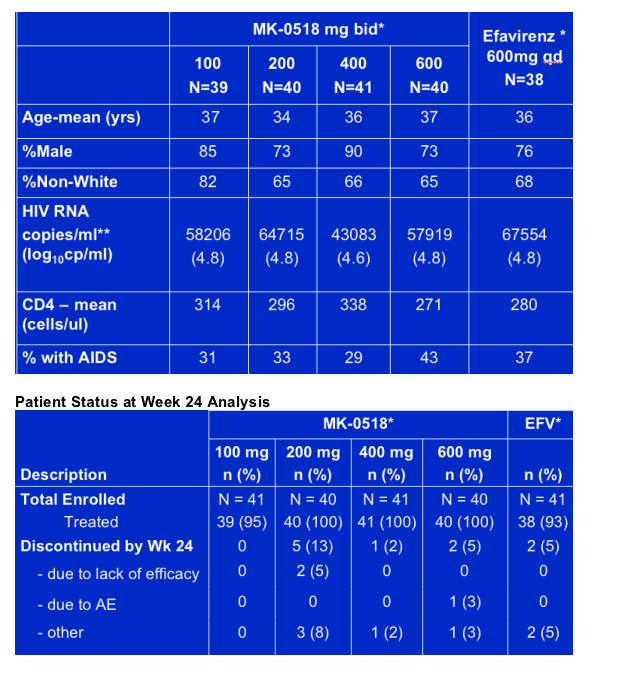
Baseline Patient Characteristics
80-90% were men; 35% were non-white; viral load was 43,000 to 64,000; mean CD4 count was 270-330; 30-43% had AIDS.
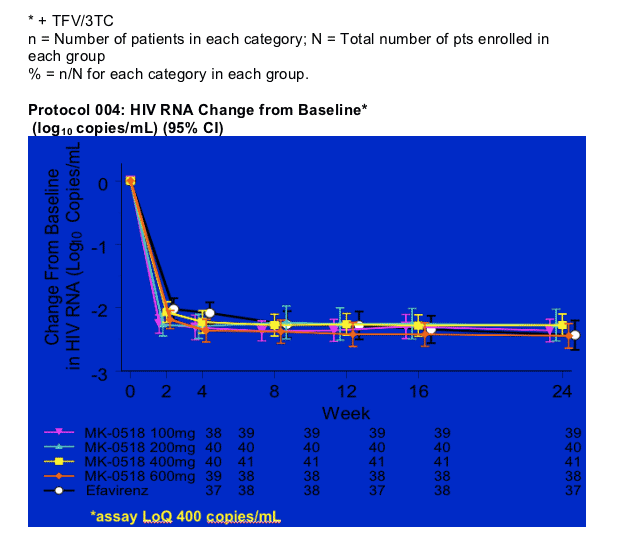
Percent (95% CI) of Patients with HIV RNA < 50 copies/mL (NC=F)
90-95% of patients receiving higher doses of MK-0518 achieved <50 copies/ml at week 24 as 92% of patients receiving efavirenz achieved <50 copies/ml. The low dose MK-0518 group had an 87% achieving <50 copies/ml. Of particular note, you can see in the graph that at weeks 4 and 8 MK-0518 achieving reducing patients viral load more quickly than efavirenz and the differences between MK-0518 and efavirenz at weeks 4 and 8 were statistically significant. This translates it appears to a high degree of potency and perhaps durability.
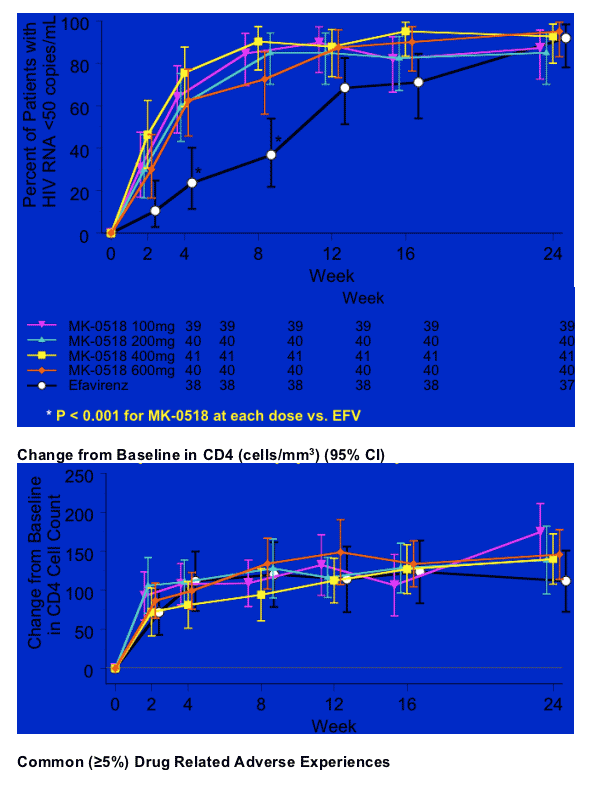
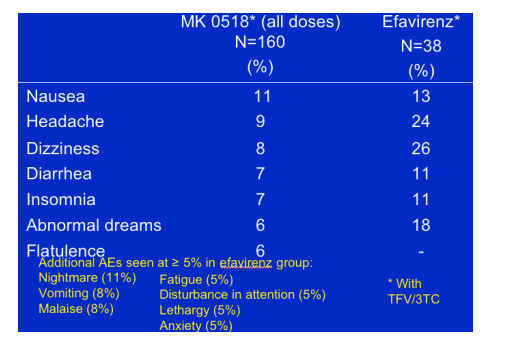
Safety
MK-0518 safety profile was similar to efavirenz (both with TFV/3TC)
-- Most clinical adverse experiences (AE) were mild to moderate
-- 8 serious adverse experiences overall (7/160 or 4% in the 4 MK groups, 1/38 or 3% in EFV group); none considered drug related
-- One discontinuation for increased AST/ALT
-- Grade 3 / 4 lab abnormalities uncommon
|
| |
|
 |
 |
|
|