 |
 |
 |
| |
TMC120 Vaginal Ring Promising As Microbicide Carrier
|
| |
| |
See IAC abstract and slides below.
By Michael Smith, Senior Staff Writer, MedPage Today
Reviewed by Zalman S. Agus, MD; Emeritus Professor at the University of Pennsylvania School of Medicine.
August 17, 2006
www.medpage.com
TORONTO, Aug. 17 -- An intravaginal ring, similar to those used to deliver contraceptives and hormonal agents, is a promising way to deliver anti-HIV microbicides, investigators reported here.
In a preliminary placebo-controlled double-blind study, a silicone elastomer ring, with a core containing an investigational HIV medication, released the drug at therapeutic levels and a constant rate in the vaginal tract for several days, they said at the 16th International AIDS Conference.
While most research on microbicides has focused on topical gels, a ring-based medication would "potentially reduce the compliance burden" on the women who use it, since it would not have to be applied at or near the moment of sexual intercourse, said Joe Romano, Ph.D., of the International Partnership for Microbicides of Silver Spring, Md., and colleagues.
The value of microbicides in the fight against HIV/AIDS was a running theme at the conference, with Microsoft billionaire Bill Gates, among others, suggesting they might be a key weapon that could turn the tide.
The medication tested in the rings is TMC120, or daviripine, a non-nucleoside reverse transcriptase inhibitor that is being developed by the Belgian pharmaceutical company Tibotec, Dr. Romano said. The company and his jointly supported the study.
"It's the concept that we are trying to see whether it's acceptable (to women) and safe," said Jens Van Roey, M.D., of the Belgian company. A next step -- and one that would likely require a large clinical trial -- would be to see whether the product actually protects women against HIV infection.
"We can't say it works," Dr. Van Roey said. "That will have to wait for Phase III trials."
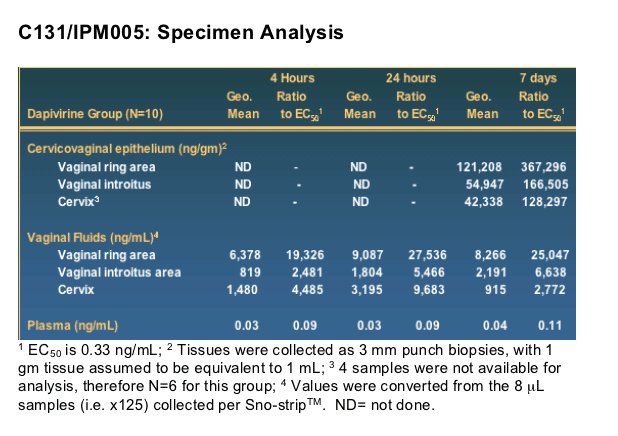
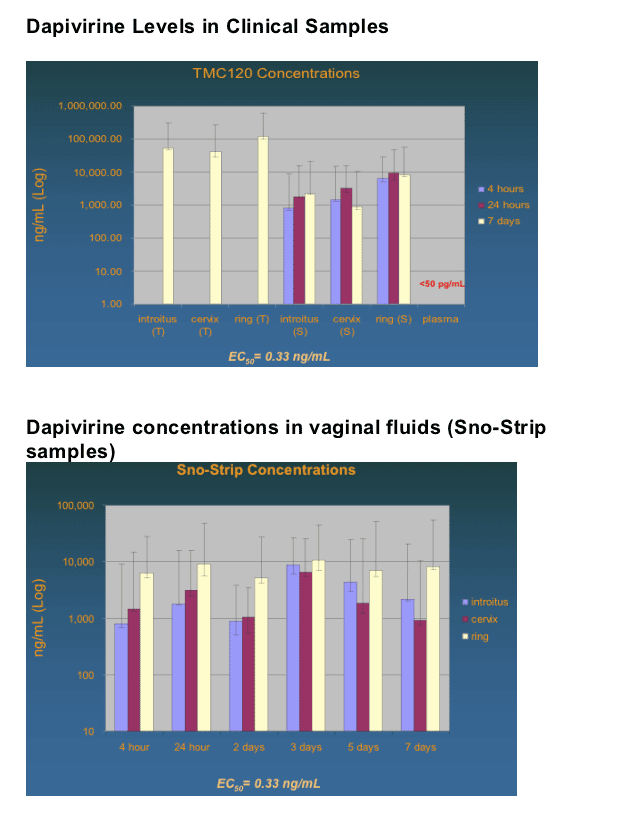
But in the small study reported here, Dr. Romano said, the ring, tested for seven days, consistently released medication at 90 times the so-called EC50 -- the concentration that produces 50% of the maximum desired effect in vivo.
However, the medication was found at high levels in vaginal fluids and tissues near the ring -- the introitus, vaginal wall, and endocervix -- but not in the plasma, where it was barely detectable at a level of just over five picograms per milliliter, he said.
In the study, 13 women had a vaginal ring with TMC120 and three had a placebo ring. The safety profile was good, Dr. Romano said:
* There were no consistent and clinically relevant changes in laboratory parameters.
* There were no clinically relevant changes in urinalysis or vital signs.
* There were no major changes in vaginal ecology pH.
One women had a cervical uterine ulcer discovered on day 14 of the study (a week after the rings were removed) but Dr. Romano said the investigators do not think it is drug-related.
There were also six cases of Grade 1 vaginal bleeding after removing the rings, four in the treatment group and two in the placebo arm. The events might have been related to oral contraceptive use, Dr. Romano said.
The rings were safe and well-tolerated, he concluded.
While most research so far has focused on microbicide gels or creams, "there will not be one tool that all men and women will like," commented Lut Van Damme, M.D., Ph.D., of the Contraceptive and Research and Development Program, based at the Eastern Virginia Medical School in Arlington.
But she cautioned that so far no microbicides have been shown to work to block HIV infection. "Once the proof of concept has been established, then it will be necessary to fine-tune," she said.
When that time comes, she added, the vaginal ring will be "a very interesting approach" because it would allow women to be defended at all times against HIV infection, in stead of needing to be applied just before sex.
Abstract
Characterization of in vitro release and in vivo delivery of TMC120 with an intravaginal ring: implications for microbicide delivery
Romano J.1, Coplan P.1, Mitchnick M.1, Douville K.1, Malcolm K.2, Van Roey J.3, Temmerman M.4, Van Bortel L.4, Weyers S.4, Rosenberg Z.1
1International Partnership for Microbicides, Silver Spring, Maryland, United States, 2Queen's University, Belfast, United Kingdom, 3Tibotec BVBA, Mechelen, Belgium, 4Universiteit Gent, Gent, Belgium
Background: The conventional approach for vaginal delivery of microbicides for the prevention of HIV transmission is administration of semi-solid formulations with an applicator. An alternative approach that achieves longer term sustained release of a microbicide is the intra-vaginal ring (IVR). TMC120 is a non-nucleoside reverse transcriptase inhibitor currently in Phase I/II trials as a microbicide in a gel formulation.
Methods: In preliminary studies, TMC120 was formulated into alternative silicone elastomer reservoir type IVR's, and was characterized for release of drug in vitro in different dissolution media. Clinical evaluation of safety and delivery was achieved in a randomized, double-blinded placebo controlled 7 day phase I study of an IVR containing 25 mg of TMC120. Ten women received the TMC120 IVR and 3 received placebo IVR. Standard laboratory diagnostics, vaginal ecology cultures, and pelvic examinations were conducted for safety assessment. Delivery was assessed via determination of TMC120 levels in plasma, as well as in cervical and vaginal tissues obtained by biopsy.
Results:
Overall it was shown that sustained release of drug in vitro was maintained for >30 days.
No clinically relevant changes in lab parameters or vaginal ecology or pH were observed. One clinically relevant abnormality was revealed upon pelvic exam, but was designated as probably unrelated to drug product. The majority of the adverse events were mild and were doubtful in terms of relation to the drug. No deaths, SAE's or AE's resulting in early termination occurred.
TMC120 was detected in vaginal fluids as early as 4 hours post-insertion, as determined with sno-strip collection. TMC120 levels were similar in samples from cervix, the vaginal ring area, and the vaginal introitus. Levels in plasma were below the lower limit of quantitation (5 pg/mL).
Conclusions: The study demonstrated that IVR delivery of TMC120 was safe and generally well tolerated, and release of drug in vivo could be achieved.
Characterization of In Vitro Release and In Vivo Delivery of TMC120 with an Intravaginal Ring: Implications for Microbicide Delivery
Joseph Romano, Ph.D.
IAC Toronto
August 16, 2006
C131/IPM008: Conclusions & Next Steps
Conclusions:
-- TMC 120 was delivered via a vaginal ring at high multiples of EC50
-- TMC120 was detectable, but near LLOQ (5 pg/mL) in plasma samples from TMC120 treatment group.
-- Both active and placebo rings were safe and well tolerated.
Next Steps:
-- IPM to conduct a ring acceptability study in Africa in 2006
-- Expanded safety studies with TMC120 in ring
-- Alternative ring designs
-- Development of manufacturing capability
Microbicide Delivery: Choice will be Key to Widespread Adoption of Microbicides
Diversity of delivery systems
Semisolids/Solids
-- Gels
-- Emulsions
-- Films
-- Tablets
Devices
-- Vaginal rings
-- Sponges
-- Diaphragm
Oral
Vaginal Rings
Attractive technology:
- 30+ days of drug delivery
- Potentially reduces compliance burden
- Easy to use
- "Low" cost
Unknowns:
- Acceptability in relevant populations
- Scale up manufacture
- Feasibility of multi-drug combinations
- Environmental impact
TMC120 (Dapivirine): Background/Status
- NNRTI developed by Tibotec/J&J, licensed to IPM (2004)
- Developed originally as therapeutic, 11 clinical studies conducted via oral administration
- Highly potent ARV
- Low cytotoxicity, non-mutagenic, non-teratogenic
- Easily manufactured
- Stable drug substance
- Very economical
- IP clarity
- Gel formulation development
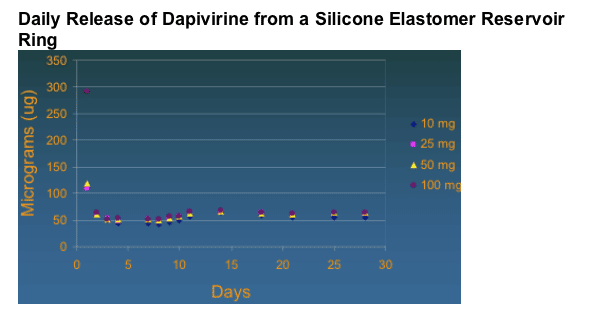
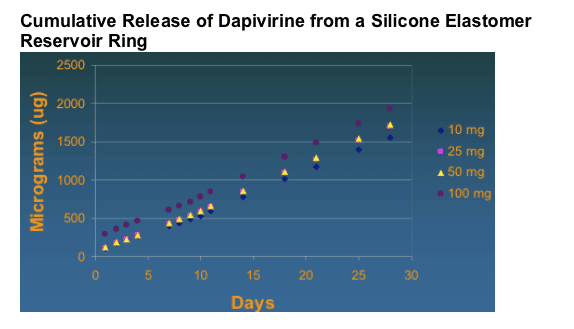
Dapivirine Formulation: Pharmacokinetics
Gel in rabbits and macaques
Tissue associated levels all much greater than EC50 even 24 hours post application
-- Good distribution of drug throughout vagina
-- Very low plasma levels
Human PK study (IPM004): Completed in Q1, 2006
Vaginal ring in humans (IPM008)
-- Good distribution of drug throughout vagina
-- Very low plasma levels
C131/IPM008: Trial Summary
Trial period: Start: 27 June 2005; End: 04 August 2005
Primary objective: Evaluate the feasibility of using a silicone elastomer reservoir type vaginal ring to deliver the candidate microbicide TMC120.
Specific objectives:
-- Assess the safety and tolerability of 7-day use of a vaginal ring containing TMC120.
-- Assess TMC120 concentrations in vaginal fluids, vaginal and cervical epithelial tissue, and plasma during and after 7-day use.
Methodology: Randomized, double blind, placebo control design
10 women with the dapivirine ring (25 mg)
3 women with placebo ring
C131/IPM008: Safety Summary
No consistent or clinically relevant changes in lab parameters were observed
One treatment emergent grade 1 lab abnormality
No clinically relevant changes in urinalysis or vital sign parameters
No mean changes in vaginal ecology pH
Nugent scores unchanged between screen and Day 14 in 9/10 women in drug arm (1 improved); no change in 2/3 in placebo arm (1 worsened)
One clinically relevant abnormality on pelvic exam (cervical uterine ulcer on day 14)
Doubtful as drug related
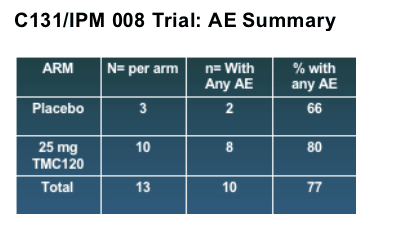
Majority of AE's were grade 1 (mild); 3 subjects with grade 2 (moderate) which were doubtful as related to drug.
Four subjects with AE's possibly related to drug; none were considered probable or very likely related to drug .
No deaths, SAE's, or AE's leading to premature discontinuation were recorded.
Drug Release: Specimen Type/Schedule
Plasma
- Blood draw: 4 hrs, 24 hrs, 7 days (pre)
Vaginal fluid
- Sno-strips: 4 hrs, 24 hrs, 7 days (pre)
-- Subgroups at 2, 3, and 5 days
Tissue
--Biopsies: 7 days (post)
Introitus
Vaginal wall
Endocervix
|
| |
|
 |
 |
|
|