 |
 |
 |
| |
CD4 Increases in Patients with Ongoing Viral Replication while Receiving Enfuvirtide are Associated with V38 and other Mutations in gp41
|
| |
| |
Reported by Jules Levin
XVI IAC Toronto, Aug 2006
Tom Melby1*, Mike DeSpirito1, Ralph DeMasi1, Gabrielle Heilek-Snyder2, Jim Thommes3,
Michael Greenberg1 and Neil Graham1
1Trimeris, Inc., Morrisville, NC, USA; 2Roche, Palo Alto, CA, USA, 3 Roche, Nutley, NJ USA
poster THPE0092
Background
Enfuvirtide (ENF, formerly T-20) inhibits HIV membrane fusion to CD4 cells by binding to viral gp41. ENF plus an optimizedbackground (OB) regimen has been shown to lead to greater gains in CD4 cell counts than those receiving OB-alone [1].
Mutations conferring resistance to ENF have been observed following virological rebound and are primarily localized within the ENF binding region on gp41, at amino acid positions 36-45; though mutations in other regions of gp41 may also emerge.
Primary resistance mutations to ENF have been shown to confer reduced viral fitness both in vitro and in vivo [2, 3] and have also been shown to result in decreased fusogenicity of viruses in vitro [4]. Viral fusogenicity has also been shown to be a primary determinant of the rate of CD4 cell decline in macaque SHIV models, even independent of viral load levels [5, 6].
Introduction
ENF has often been used in treating patients with few or no other active antiretrovirals, potentially facilitating virological breakthrough. Due to a lack of alternative treatment options, ENF dosing is often continued after viral load rebound and appears to result in continued CD4 cell benefits in some patients [7, 8].
Patients failing an ENF-based regimen with viruses harboring the most commonly observed ENF resistance mutation, V38A (or V38E, n=15), had a continued CD4 increase at 24 weeks while a decline was observed for patients with viruses developing the less common Q40H+L45M double mutation (n=5) [9].
Given the impact of these mutations on viral fusogenicity and the established correlation between fusogenicity and magnitude of CD4 cell declines in animal models, we explored this association in a larger dataset.
Author Discussion and Conclusions
Important highlights of these analyses included the following:
Specific mutations and CD4 gains:
- At Week 48, CD4 gains over baseline persisted for all groups of patients, but were greatest where V38 mutations emerged and were weaker in patients developing the N43 or Q40 mutations.
- The Q40 mutation was associated with a relatively large early increase in CD4 count followed by a steady decline after virological rebound. In contrast, patients with viruses developing N43 mutations experienced more moderate initial CD4
increases but largely maintained their CD4 cell gains after virological rebound. Thus, only Q40 mutations were clearly detrimental to continued CD4 benefits, as shown previously [9, 10].
Mechanism and role of viral suppression:
- Continued viral suppression did not fully explain the differences between mutation groups, suggesting a differential impact of various mutations on viral pathogenicity for CD4 cells.
- This is consistent with in vitro studies of the impact of specific ENF resistance mutations on viral fusogenicity and of viral fusogenicity on CD4 cell declines in animal models [4-6].
- One potential mediator of these effects could be altered activation levels associated with ENF-based therapies [8].
Factors to consider when interpreting these results:
- The patients studied here represent only those who, despite virological rebound, continued dosing with ENF through 48 weeks (or, in one analysis, for 48 weeks after virological failure). However these included a majority (> 70%) of eligible patients, suggesting that they should be at least broadly representative of
other groups of patients.
- Viral genotypes may have evolved between the time of testing and the time of CD4 change analysis. Additional sequencing will be necessary to allow assessment of CD4 change and gp41 genotype at the same time.
- These analyses did not fully address the relative contribution of partial viral load suppression and altered pathogenicity for CD4 cells.
RESULT
Of 661 patients randomized to receive ENF plus an OB regimen; 269 had genotypic data available and met virological failure criteria prior to Week 40; 193 remained on treatment at Week 48.
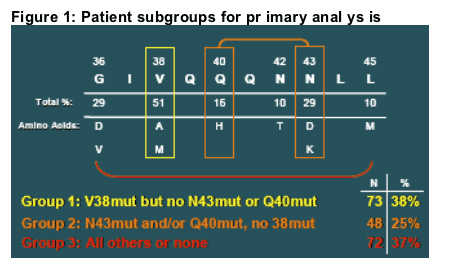
At the time of virological failure (when resistance testing was performed) 38% of these patients had mutations at position 38 but not at 40 or 43; 25% had mutations at positions 40 and/or 43 but not 38; and 37% had mutations either at combinations of these positions, only at other positions, or in a few cases, no mutations.
Baseline Characteristics
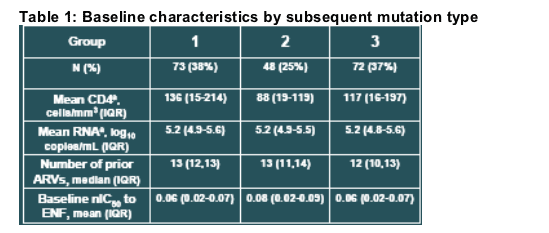
At baseline, there were no significant differences between the groups of patients who subsequently developed the different types of mutations. However, the lowest baseline CD4+ cell count was observed for the group which subsequently experienced the least improvement in CD4 counts on treatment (Table 1).
CD4 cell response during treatment
A significantly greater increase in CD4 cells from baseline was observed for patients developing mutations at gp41 position V38 (95 cells/mm3) than for those with mutations at positions Q40 or N43 (49 cells/mm3; P=0.034). The response of patients with combinations of these mutations or other genotypes (group 3) was intermediate to the other groups (67 cells/mm3, P>0.1).
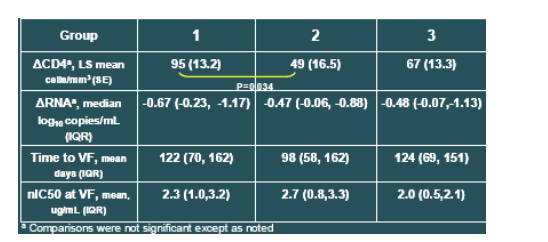
The magnitude of viral load response was comparable between the groups. Median changes in VL from baseline to Week 48 were -0.67, -0.47, and -0.48 log10 copies/mL, for Groups 1,2 and 3, respectively.
Even after adjustment for on-treatment changes in plasma HIV RNA, the observed CD4+ count differences among the three mutational groups were still evident (LS means of 90, 54, and 68 cells/mm3, for Groups 1, 2, and 3, respectively), suggesting that the CD4 effect across groups was not fully explained by differences in virologic response.
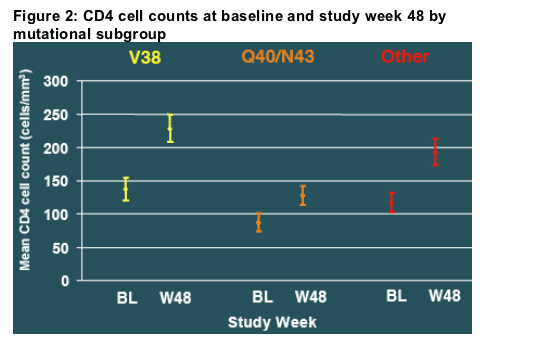
Both baseline CD4 cell count and the CD4 cell increase during treatment were lower for patients who developed Q40 or N43 than for those who developed mutations at position V38. (Figure 2)
Analysis Post-VF and response of Q40 versus N43
These analyses were extended in a subsequent analysis of CD4 count response relative to the time of virological failure (Figure 3) [10]. This analysis examined Q40 and N43 patients separately and also adjusted for virological response (and other factors) using analysis of covariance (ANCOVA).
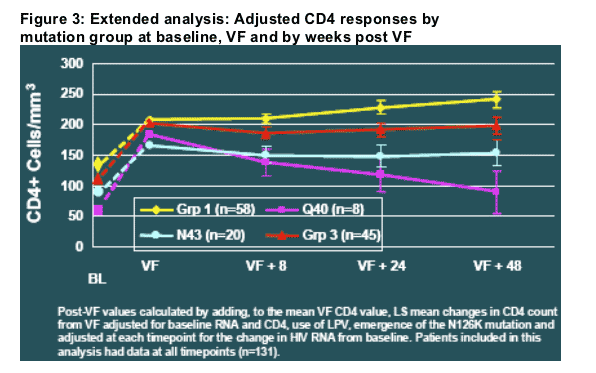
Figure 3 illustrates the following points:
- The differences in CD4 response between mutation groups was evident even 48 weeks after the time of virological failure.
- The differences were evident after adjusting for differences in virological response between the groups.
- From the time of virological failure forward, N43 mutations were associated with a relatively stable CD4 benefit, whereas the Q40H mutation was associated with a steady loss of the CD4 cell benefit observed for this group at the time of VF.
METHODS
Patient Populations
Patients who were randomized to receive ENF plus an OB regimen in ENF Phase 3 studies; met virological failure (VF) criteria prior to Week 40, continued therapy with an ENF-based regimen through Week 48, and had data on change in CD4+ cell count, viral load and envelope genotype were eligible for these analyses.
An additional analysis (shown in Figure 3) included patients with VF through study week 48; genotype at baseline and VF; and CD4 count data at 8, 24 and 48 weeks after VF (up to study week 96; n=131; data presented previously [10]).
Subgroup Determinations
Patients were subgrouped based on viral envelope genotype in gp41 amino acids 36-45 (gp160 residues 547-556) at the time of meeting VF criteria (Figure 1).
They were classified by the presence of a substitution at gp41 position V38 but not Q40 or N43 (38%); Q40 and/or N43 but not V38 (25%, combined due to comparable responses at study week 48); or for all other genotypes (37%) (including patients with combinations of these mutations, with G36D or N42 mutations, or with no mutations).
For Figure 3, the groupings were similar except that patients with Q40 versus N43 mutations were shown separately and patients with both of these mutations were excluded from the analysis.
Statistical Analyses
Least squares (LS) mean CD4 changes were calculated and compared using analysis of covariance (ANCOVA) adjusting for:
- Baseline CD4
- Study day of virological failure
- The number of antiretrovirals used prior to the study
Continuous variables were compared by the Wilcoxon two-sample test with two-sided p-values: >0.1 non-significant (ns); 0.01-0.05 of borderline. Susceptibility to ENF was summarized as the normalized IC50 value, which is the IC50 multiplied by the ratio of a standard IC50 value obtained for the reference over multiple assay runs to the IC50 for the reference strain run concurrently with the sample [11].
|
| |
|
 |
 |
|
|