 |
 |
 |
| |
Risk of discontinuation of nevirapine due to toxcities in antiretroviral naive and experienced patients with high and low CD4 counts
|
| |
| |
Reported by Jules Levin
XVI IAC Toronto, Aug 2006
Mocroft A.1, Lacombe K.2, Rockstroh J.3, Gasiorowski J.4, Antunes F.5, Panos G.6, d'Arminio Monforte A.7, Rakhmanova A.8, Phillips A.N.1, Lundgren J.D.9, for the EuroSIDA study group
1Royal Free and University College Medical School, Primary Care and Population Sciences, London, United Kingdom, 2Saint-Antoine Hospital, Paris, France, 3Universitäts Klinik, Bonn, Germany, 4Medical University, Warsaw, Poland, 5Hospital Santa Maria, Lisbon, Portugal, 61st IKA Hospital, Athens, Greece, 7Osp. L. Sacco, Milan, Italy, 8Medical Academy Botkin Hospital, St Petersburg, Russian Federation, 9Hvidovre University Hospital, Hvidovre, Denmark
authors found: ".....While NVP is not recommended for antiretroviral-naive patients with high CD4 counts due to the risk of TOXPC, results from this analysis suggest it may be safer for use in antiretroviral experienced-patients starting NVP...."
Background: There is an increased risk of toxicities in antiretroviral-naive patients starting nevirapine-based cART (NVPc) with high CD4 counts. It is not known whether this risk applies to other patient groups starting NVPc.
Methods: EuroSIDA patients starting NVPc after 1 January 1999 with CD4 and viral load measured in the 6 months before starting cART were included. CD4 at starting NVPc was classified as high (>400/mm3/>250/mm3 male/female respectively) or low. Cox proportional hazards models were used to compare the risk of discontinuation of nevirapine due to toxicities or patient/physician choice (TOXPC) in 4 groups; antiretroviral-naive/low CD4 (LN), antiretroviral-experienced/low CD4 (LE), antiretroviral-naive/high CD4 (HN) and antiretroviral-experienced, high CD4 (HE).
Results:
1484 patients were included; of these, 28/138 (20.3%) in the LN group, 153/581 (27.3%) in LE, 31/65 (47.7%) in HN and 195/720 (27.1%) in HE discontinued nevirapine due to TOXPC (p=0.007). The figure shows the univariate and multivariate relative hazard of discontinuation of nevirapine due to TOXPC; HN was the reference group.
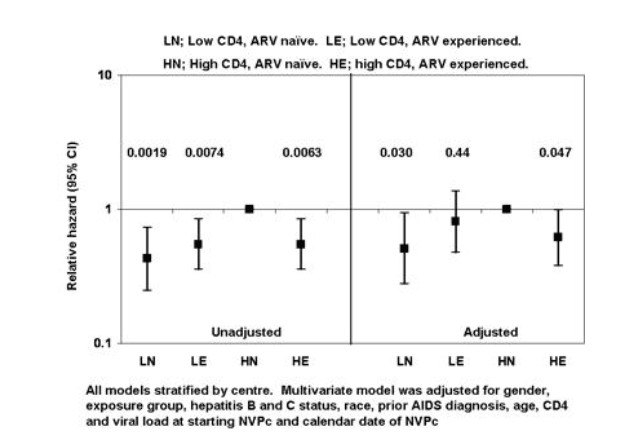
[figure 1] In analyses using identical groups, including patients starting either efavirenz-based cART (n=2114) or protease-inhibitor based cART (n=1522), there were no differences in risk of discontinuation due to TOXPC when comparing HN and HE groups after adjustment (p=0.33 and 0.95 respectively).
Conclusions:
Patients in the HE group had a reduced risk of discontinuation due to TOXPC compared to patients from the HN group. Patients in this study were not randomised to treatment, and the results should be interpreted with caution. While NVPc is not recommended for antiretroviral-naive patients with high CD4 counts due to the risk of TOXPC, results from this analysis suggest it may be safer for use in antiretroviral experienced-patients starting NVPc.
|
| |
|
 |
 |
|
|