 |
 |
 |
| |
TMC114/r in treatment-experienced HIV patients in POWER 3: 24-week
efficacy and safety analysis
|
| |
| |
Reported by Jules Levin
XVI IAC Toronto, Aug 2006
Molina JM,1 Cohen C,2 Katlama C,3 Grinsztejn B,4 Timerman A,5 Pedro R,6 De Meyer S,7 de Béthune M-P,7 Vangeneugden T,7 Lefebvre E8
1Hôpital St-Louis, Division of Clinical Microbiology and Infectious Diseases, Paris, France; 2Community Research Initiative of New England, Boston, MA, USA; 3Hôpital Pitié-Salpêtrière, Paris, France;
4Institut De Pesquisa Clînica Evandro Chagas, Rio de Janeiro, Brazil; 5PAM Heliopolis, São Paulo, Brazil; 6HC Unicamp, Campinas, Brazil; 7Tibotec BVBA, Mechelen, Belgium; 8Tibotec Inc., Yardley, PA, USA
poster TUPE0060
ABSTRACT
Background: In the POWER 1 and 2 studies (TMC114-C213 and TMC114-C202), the protease inhibitor (PI), TMC114 (darunavir), co-administered with low-dose ritonavir (TMC114/r), provided significantly greater viral load (VL) reduction and CD4 increase than control PIs (CPIs). The efficacy and safety of the recommended dose for treatment-experienced patients, 600/100mg bid, were further investigated in the nonrandomized, open-label POWER 3 analysis (TMC114-C215/C208).
Methods: Study inclusion/exclusion criteria were the same as for POWER 1 and 2. Patients in POWER 3 received TMC114/r 600/100mg bid plus an optimized
background regimen (OBR; NRTIs ± enfuvirtide [ENF]). Analysis was by intent-to-treat (ITT) (time-to-loss of virologic response [TLOVR] algorithm).
Results: Of the 327 patients enrolled, the efficacy analysis included 246 patients
who reached Week 24; the safety analysis included all patients. Baseline (BL)
characteristics were similar to those of POWER 1 and 2: mean VL was
4.6 log10 copies/mL and median CD4 count was 115 cells/mm3. HIV RNA
<50 copies/mL and at least a 1 log10 copies/mL
HIV RNA reduction were achieved by 40% and 65% of patients, respectively. BL TMC114 fold change in EC50 (FC) was the strongest predictor of virologic response. CD4 counts increased by a mean of 80 cells/mm3.
The most common adverse events (AEs) were diarrhea (14%), nasopharyngitis (11%) and nausea (10%). Grade 3/4 triglyceride, cholesterol, alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) elevations occurred in 6%, 4%, 2% and 2% of patients, respectively.
Most of the AEs leading to discontinuation (in eight patients [2%]) did not occur in >1 patient. No grade 3/4 AEs (regardless of causality) occurred in >4% of patients. No serious AE (SAE) occurred in >1% of patients. The six deaths (2%) were not treatment-related.
Conclusions: POWER 3 efficacy and safety results confirm and extend those observed in POWER 1 and 2 in a larger population. TMC114/r 600/100mg bid provided patients with a substantial VL reduction and CD4 cell increase, and was generally safe and well tolerated.
- POWER 3 is an open-label, non-randomized analysis, which was conducted to assess the long-term efficacy and safety of TMC114/r 600/100mg bid in HIV-1-infected, treatmentexperienced patients.
- This analysis included patients who initiated treatment with TMC114/r 600/100mg bid plus an investigator-selected OBR (32 NRTIs with or without ENF) in the TMC114-C215 and TMC114-C208 trials.
RESULTS
BL characteristics
- All 327 patients who enrolled were included in the safety analysis; 246 patients who reached Week 24 or discontinued earlier were included in the efficacy analysis.
- At BL, mean VL was 4.6 log10 copies/mL and median CD4 count was 115 cells/mm3 (Table 1).
- Patients had a median of three primary PI mutations (IAS-USA March 20058); consequently, only 20% of patients had HIV which was sensitive to another PI at screening (based on Antivirogram data) excluding tipranavir (TPV), which was not available at the time of recruitment. Among the enrolled patients, >/=99% had used >/=1 PI, 98% >/=4 NRTIs and 98% >/=1 NNRTI, similar to patients from POWER 1 and 2.
Overall efficacy at Week 24
- Overall efficacy results were similar to those obtained in POWER 1 and 2.
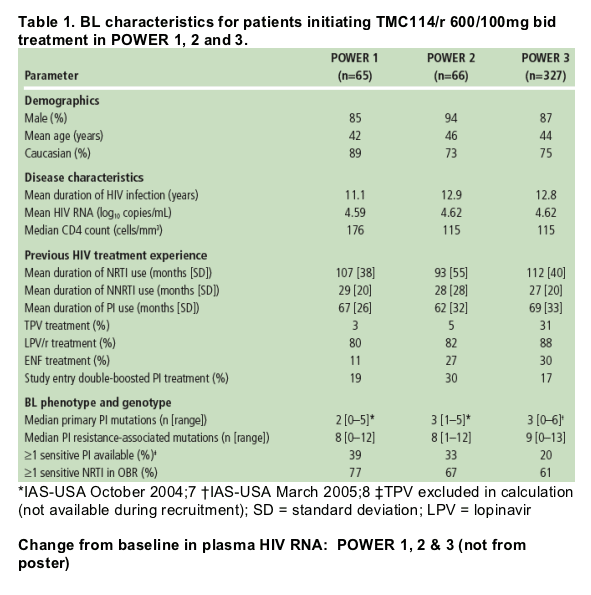
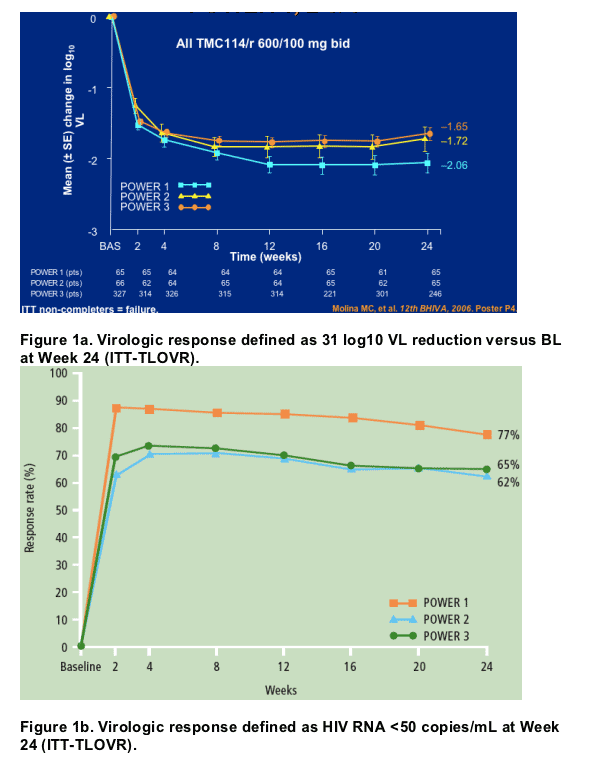
- The primary efficacy endpoint of >/=1 log10 VL reduction was observed in 65% of patients (Figure 1a).
- VL reduction to <50 copies/mL was seen in 40% of patients (Figure 1b).
- Mean VL reduction from BL to Week 24 was -1.65 log10 copies/mL.
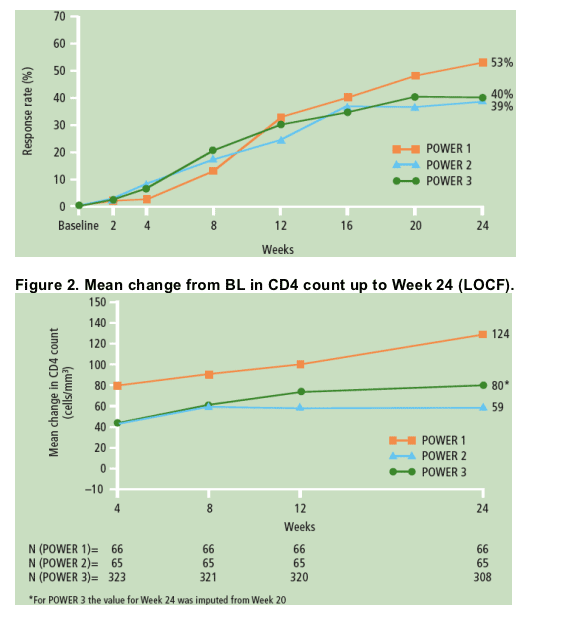
- CD4 counts at Week 24 (last observation carried forward [LOCF]) increased by a mean of 80 cells/mm3 (Figure 2), similar to the mean CD4 increase at Week 20 in TMC114/r patients (90 cells/mm3) from the integrated analysis of POWER 1 and 2.
Factors influencing treatment response
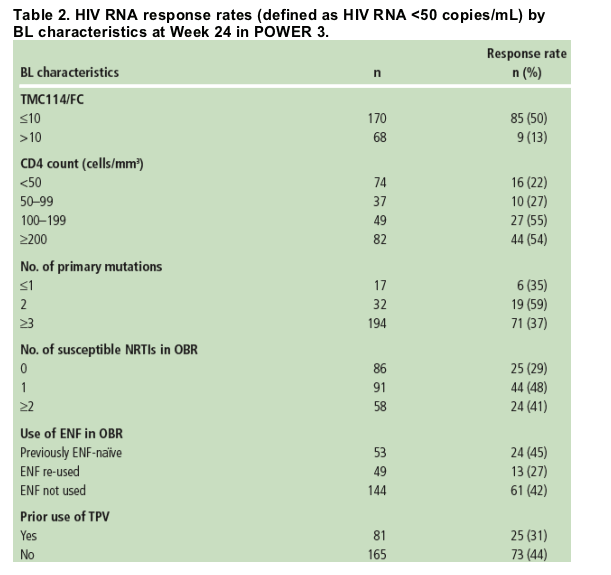
- The impact of BL factors on treatment response at Week 24 is shown in Table 2.
- In a multivariate analysis, BL phenotypic TMC114 FC was the strongest predictor of response, which was also demonstrated in POWER 1 and 2.10 A higher proportion of patients achieved VL <50 copies/mL with a BL TMC114 FC >/=10 (50%) compared with patients with FC >10 (13%).
- There was no clear relation between the number of primary PI mutations at BL and virologic response.
- Higher rates of virologic suppression (VL <50 copies/mL) were achieved by those who had >/=1 susceptible NRTI in their OBR (46%) compared with those who had no susceptible NRTI in their OBR (29%).
- According to pooled data of POWER 1, 2 and 3 patients who initiated treatment with TMC114/r 600/100mg bid, 24% had previously used TPV. BL resistance to TPV (using a clinical cut-off of 3.0) was associated with an increase in TMC114 FC. However, the observed change in VL of -1.38 log10 copies/mL for TPV-resistant patients was close to the overall value of -1.74 log10 copies/mL for all patients who initiated treatment with TMC114/r 600/100mg bid.
Safety
- TMC114/r was generally well tolerated and no specific toxicity was associated with TMC114/r treatment.
- The most common treatment-emergent clinical AEs were diarrhea (14%), nasopharyngitis (11%) and nausea (10%) (apart from injection-site reaction, which occurred in 19% of all patients; this AE was observed only in ENF-treated patients).
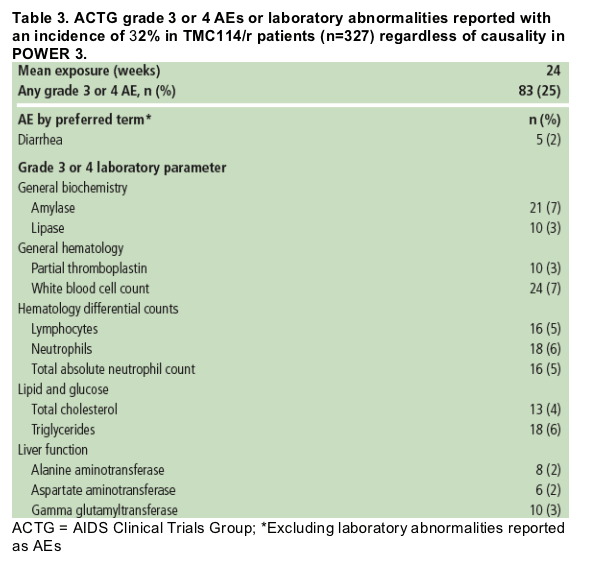
- The majority of AEs were grade 1 or 2 in severity; 25% of patients reported >/=1 grade 3 or 4 AE. Grade 3 or 4 AEs occurring in >/=2% of patients are displayed in Table 3. Treatment-emergent grade 3 or 4 lipid elevations were uncommon and none led to withdrawal (4% chol, 6% TG); grade 3 or 4 liver-related abnormalities were also uncommon.
- The overall incidence of SAEs was 13%; no individual SAE occurred in >1% of patients. All SAEs reported during POWER 3 occurred in one patient each, except for pneumonia and metabolic acidosis (each reported in three patients), and vomiting, pancytopenia, pyrexia and renal insufficiency (each reported in two patients). None of the six deaths (2%) was considered related to TMC114/r treatment.
- Overall, 8% of patients discontinued. Notably, there was a low rate of treatment
discontinuation due to AEs or HIV-related events (2%) and due to virologic failure (2%). Most of the AEs leading to discontinuation did not occur in more than one patient.
- Mean changes in lipid laboratory parameters between BL and Week 24 were small, with mean changes from BL of -0.34, 0.43 and 0.42mmol/L recorded for triglycerides, total cholesterol and low density lipoprotein, respectively.
- According to pooled data of POWER 1, 2 and 3 patients who initiated treatment with TMC114/r 600/100mg bid, there was a pronounced decrease in triglycerides at Week 24 for patients who had received LPV/r during screening then received TMC114/r during the study period, compared with those who had not received LPV/r. No obvious differences in changes from BL in other lipid parameters were observed between these two subgroups of patients.
Author Conclusions
- The efficacy findings from POWER 3 extend and confirm the observations from POWER 1 and 2. TMC114/r 600/100mg bid provides sustained VL reductions and CD4 increases in treatment-experienced patients.
- Together with POWER 1 and 2, POWER 3 results clearly demonstrate that VL suppression to undetectable levels can be achieved for many treatment-experienced patients.
- The safety results from the analysis confirm the findings from POWER 1 and 2, and further indicate that TMC114/r 600/100mg bid is generally well tolerated. No unforeseen AEs were observed in this large patient set.
- Phase III randomized controlled trials of TMC114/r are ongoing in treatment-naive (ARTEMIS [TMC114-C211]) and treatment-experienced patients (TITAN [TMC114-C214]).
|
| |
|
 |
 |
|
|