 |
 |
 |
| |
Patient Survey: Fuzeon Biojector Improves Pain & ISRs, Adherence, Injection Concerns, and Interference with Daily Activities; 75% prefer Biojector to the standard needles/syringes
|
| |
| |
Reported by Jules Levin
XVI IAC Toronto, Aug 2006
"An Ongoing Nonrandomized Large Prospective Evaluation of Alternative Injection Devices (Biojector B2000, Standard Needles/Syringes, or Insulin Needles/Syringes) for Enfuvirtide in a National Community-Based Specialty Pharmacy"
S. Tschida, A. Zappa, M. Godwin
BioScrip Pharmacy, Eden Prairie, MN, USA
The objective of this study:
To evaluate the impact of the Biojector, standard 27G 1/2" syringes, and shorter
thinner gauge needles/syringes (i.e., insulin syringes) on the persistence/adherence, tolerability, acceptability and attitude towards injection, and preference/satisfaction with ENF in patients using a large national specialty pharmacy.
To date, 1119 patients have been enrolled into the utilization review: 1042 Biojector patients, 49 insulin syringe patients, and 39 standard syringe patients.
KEY SUMMARY POINTS:
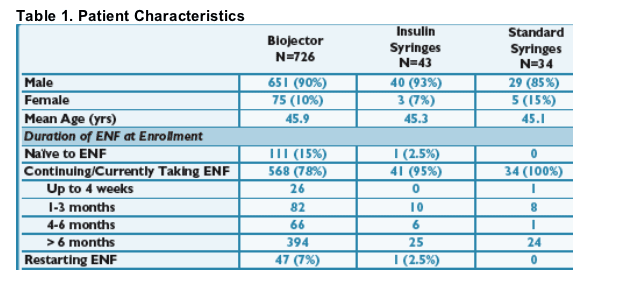
For ENF na´ve patients, the most common reason for starting the Biojector was needle phobia. For those continuing/currently taking ENF, the most common reasons were needle fatigue followed by moderate-to-severe ISRs. For those restarting ENF, the most common reasons were needle fatigue followed by needle phobia.
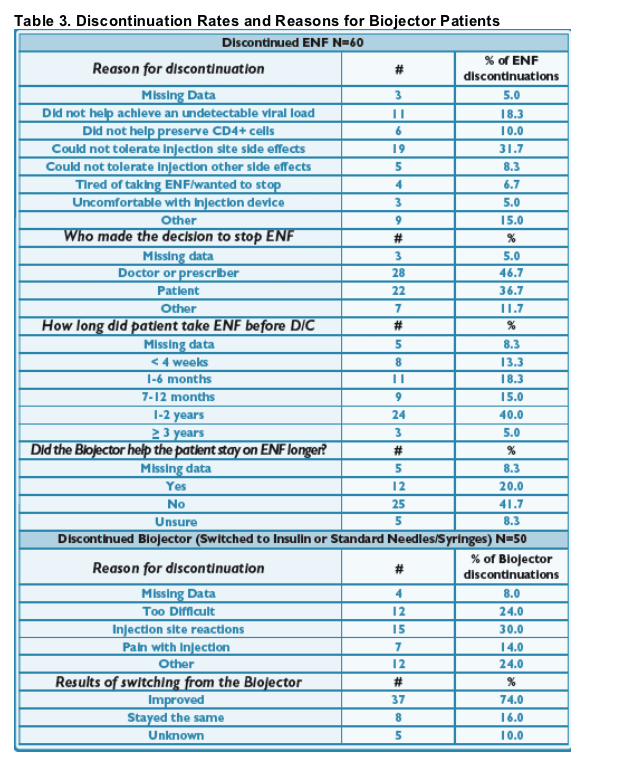
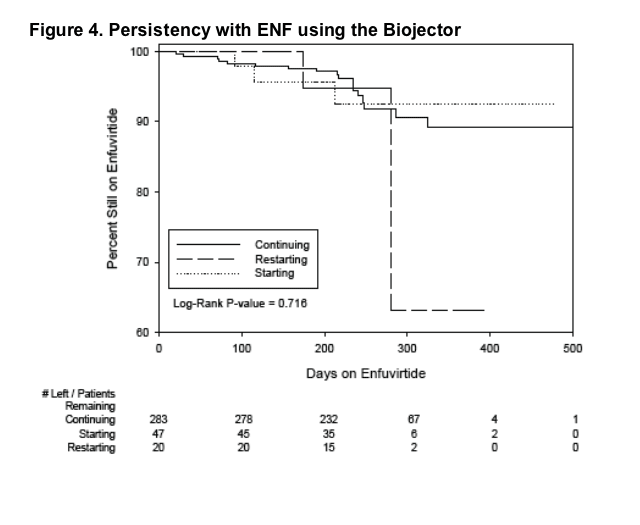
92% (666/726) of ENF patients who started the Biojector remain on ENF; primary
reasons for discontinuing ENF included lack of virologic control (17/726, 2%) and ISRs (19/726, 3%). 8% discontinuation rate. (See Tables 2 & 3)
93% (616/666) of continuous ENF patients who started the Biojector continue on it. Of those that discontinued using the Biojector, 56% (28/50) switched to standard needle/ syringe and 36% (18/50) to insulin syringes.
Adherence
- The frequency of nonadherence by refill metrics (MPR* < 0.85; <85%) appears similar between Biojector and insulin syringe patients. See Table 4.
- Overall ENF adherence with Biojector by refill metrics was good with a mean MPR of 0.89. (89%)
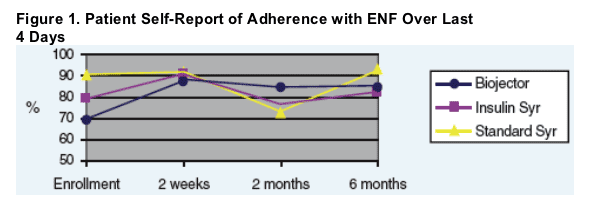
- ENF adherence based upon patient report, "ENF schedule was followed most/all of time" for Biojector patients, improved 23% by 6 months. (see Figure 1 below)
Tolerability and Attitude/Preference
For patients that switched to the Biojector, pain and ISR symptoms were significantly improved at each assessment period from baseline (p<0.05). Injection fear, concern, difficulty, and interference on daily activities were also significantly improved at each time period from baseline (p<0.01). See Figure 3.
For patients switched to the Biojector, ENF injections interfered with their daily activities 40% of the time prior to the switch but only 25% and 17% by 2 and 6 months after the switch to Biojector, respectively.
Of the 165 patients evaluated at 6 months, 123 (75%) preferred the Biojector to the standard needles/syringes.
Author Conclusions:
- The Biojector demonstrated high levels of persistency, tolerability, and patient
acceptability.
- Improvements in tolerability and patient acceptibility were significant and most
pronounced in patients switched from other injection methods to the Biojector. This is our largest cohort of patients and the majority (54%) of these patients had been on ENF for > 6 months before switching to the Biojector.
- Overall, the rate of ENF discontinuation in the Biojector group was only 8.3% and often due to reasons not directly impacted by the Biojector device.
- Overall ENF adherence with Biojector by refill metrics was good with a mean MPR of 0.89. Adherence by patient report improved 23% by 6 months with the Biojector.
- For people switched to the Biojector, tolerability (pain and other injection reactions) significantly improved. Injection fear, concern regarding ISRs, difficulty giving injection, and interference of injection on daily activities were also significantly improved.
- A high percentage (75%) of people preferred the Biojector to needles/syringes for administration of ENF.
Background
Injection site reactions (ISRs) and needle fatigue occur frequently with administration of enfuvirtide (T-20, Fuzeon) and may contribute to delayed enfuvirtide (ENF) initiation and/or early discontinuation. Needle-free administration with the Biojector B2000 (Biojector) may offer a viable alternative to the standard 27G 1/2" needle/syringe or a shorter gauge needle/syringe for ENF and improve tolerability increase persistence with therapy and
reduce ISRs. In Canada, use of the Biojector for ENF administration is being studied in two trials. An analysis of 23 patients with a median ENF duration of 8 months who were switched to the Biojector after having difficulty with the standard needle/syringe showed a significant decrease in the number and scoring (severity) of ISRs.1 Patients also rated the Biojector as significantly easier to use. The second study has reported data from 105 patients with
a median ENF duration of 8 months.2 Administration of ENF using the Biojector produced a significant decrease in the scoring of ISRs. Seventy-one percent of patients preferred Biojector over the standard needle/syringe. Collectively, these recent data suggest the Biojector may increase acceptability, tolerability, satisfaction, and persistence with ENF.
STUDY DESIGN
Prospective, open label, nonrandomized, 12 month utilization evaluation of alternative injection devices (Biojector or insulin syringes) versus standard 27G 1/2" syringes for ENF administration. Distribution of and training for the Biojector is provided through a national specialty pharmacy company with 32 retail locations and a mail-order facility.
Patients are eligible for the Biojector if one of the
following criteria is met:
- Patient has moderate-to-severe ISRs
- Patient has "needle fatigue" and/or needle-related reactions resulting in risk of discontinuing ENF
- Patient is "needle phobic" or refuses to use needles/syringes
- Patient has a physical impairment and cannot use a syringe
Patients are eligible for using insulin syringes if they have ISRs of any severity.
Questionnaires assessing adherence, tolerability, attitude/satisfaction, and switching/ discontinuation of administration device are completed by patients at enrollment, 2 weeks, and 2, 6, and 12 months. ENF adherence and persistency is also measured using BioScrip Pharmacy prescription utilization data. If a patient discontinues ENF, the reasons for discontinuation and perceptions of the impact of the administration device on persistency with ENF are assessed.
Evaluation Period
Enrollment commenced February 18, 2005 and will end August 31, 2006. To date, 1119 patients have been enrolled into the utilization review: 1042 Biojector patients, 49 insulin syringe patients, and 39 standard syringe patients.
RESULTS
Analysis of Patients Enrolled During the Initial 10 Months of Program:
Of the 803 patients enrolled during this period, 553 had completed the 2 week survey, 437 completed the 2 month survey, and 203 had completed the 6 month survey (172 Biojector patients, 17 insulin syringe patients, and 14 standard needle/syringe patients). 301 patients received the 6 month survey and the response rate was 67%.
Patient Characteristics
- Recruitment into the Biojector cohort far outweighed recruitment into the insulin syringe and standard syringe cohorts, limiting statistical comparison. There is some potential recruitment bias in the insulin syringe and standard syringe cohorts as many of these people were on ENF > 6 months and potentially more stable on therapy.
- For ENF na´ve patients, the most common reason for starting the Biojector was needle phobia. For those continuing/currently taking ENF, the most common reasons were needle fatigue followed by moderate-to-severe ISRs. For those restarting ENF, the most common reasons were needle fatigue followed by needle phobia.
ENF and Biojector Discontinuations
- 92% (666/726) of ENF patients who started the Biojector remain on ENF; primary reasons for discontinuing ENF included lack of virologic control (17/726, 2%) and ISRs (19/726, 3%). (See Tables 2 & 3)

93% (616/666) of continuous ENF patients who started the Biojector continue on it. Of those that discontinued using the Biojector, 56% (28/50) switched to standard needle/ syringe and 36% (18/50) to insulin syringes.
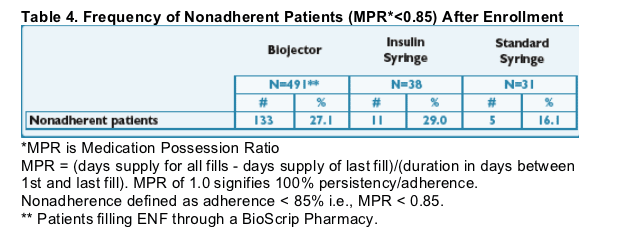
- The frequency of nonadherence by refill metrics (MPR* < 0.85) appears similar between Biojector and insulin syringe patients.
- Overall ENF adherence with Biojector by refill metrics was good with a mean MPR of 0.89.
- ENF adherence based upon patient report, "ENF schedule was followed most/all of time" for Biojector patients, improved 23% by 6 months.
Tolerability and Attitude/Preference
For patients that switched to the Biojector, pain and ISR symptoms were significantly improved at each assessment period from baseline (p<0.05). Injection fear, concern, difficulty, and interference on daily activities were also significantly improved at each time period from baseline (p<0.01).
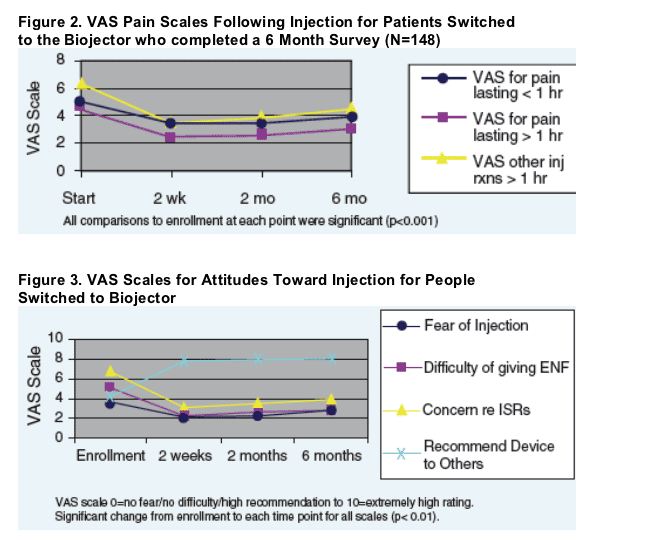
For patients switched to the Biojector, ENF injections interfered with their daily activities 40% of the time prior to the switch but only 25% and 17% by 2 and 6 months after the switch to Biojector, respectively.
Of the 165 patients evaluated at 6 months, 123 (75%) preferred the Biojector to the standard needles/syringes.
Updated Analysis of Only Patients Who Completed a 6 Month Survey (May 2006):
- Of the 1119 patients enrolled from February 2005 to May 2006, 417 have completed the 6 month data survey; 377 Biojector patients, 20 insulin syringe patients, and 20 standard needle/syringe patients. 744 patients received the 6 month survey during this period and the survey response rate was 54%.
- All data are similar to those of the initial analysis of all patients enrolled during the first 10 months of the program except the following:
- 48% of people who discontinued ENF while using the Biojector reported that Biojector helped them stay on ENF longer than if they used standard needle/ syringes. This is much higher than the previous reported rate of 20%.
- Availability of persistency data.
|
| |
|
 |
 |
|
|