 |
 |
 |
| |
Nonnukes vs PIs: Digesting the Latest Data
|
| |
| |
A report from the XVI International AIDS Conference
Toronto, August 13-18, 2006
Mark Mascolini
For most HIV clinicians in most places, whether to start someone's antiretroviral rovings with a nonnucleoside (NNRTI) or a protease inhibitor (PI) poses no deep mystery. In 90% of the world economics dictate the choice, and the omnipresence of fixed-dose nevirapine ensures the ascendance of nonnuke starting medleys. In richer countries, the potential adverse events associated with some PI combos apparently explains some clinicians' preference for first-line NNRTIs over the past several years.
All along it looked like efavirenz or nevirapine could control HIV as well as a PI--and in the case of efavirenz, at least, better than an unboosted PI [1,2]. So the first-line pick came down to questions of convenience, side effects, and personal preference. But SRO crowds at Toronto's International AIDS Conference heard the first hints that NNRTIs offer a virologic advantage over PIs. Yet, as proves true so often in these first-line frays, the outcome's not as simple as that.
Busy arithmeticians at the Antiretroviral Therapy Cohort Collaboration set the table for the Toronto entrees with a 12-cohort reckoning of new AIDS diagnoses and death according to the first antiretroviral regimen taken by 17,666 people [3]. Through 55,622 person-years at risk, statisticians counted 1617 new AIDS diagnoses and 895 deaths from any cause. Compared with up-front efavirenz, the risk of AIDS or death was 1.45 times higher in people who started a ritonavir-boosted PI (adjusted hazard radio [AHR] 1.45, 95% confidence interval [CI] 1.15 to 1.18), and the risk of having a detectable viral load 6 months after starting therapy was 1.67 times higher in the PI-plus-ritonavir group (AHR 1.67, 95% CI 1.35 to 2.07).
Of course cohort studies--even real big cohort studies--can't replace randomized trials as bases for antiretroviral planning. And the limitations of the Collaboration's NNRTI-versus-boosted PI face-off are readily apparent. Most people in the PI group took boosted indinavir (58%) or saquinavir (40%), while only 11 (1%) took lopinavir/ritonavir and 14 (1%) amprenavir/ritonavir. No one took boosted atazanavir. Furthermore, 40% of cohort members who started a boosted PI were no longer taking that regimen 6 months later. Finally, the authors observe, because prognostic factors such as pretreatment viral load and CD4 count may be mismeasured, it's possible that even the adjusted hazard ratio for progression or a detectable load at 6 months "continues to be an overestimate of the true risk associated with boosted PI regimens."
Still, the cohort results served notice that boosted PIs may be vulnerable in first-line comparisons with efavirenz.
Efavirenz trumps lopinavir virologically
AIDS Clinical Trials Group (ACTG) study A5142 entered the antiretroviral annals as the first randomized comparison of efavirenz with lopinavir/ritonavir, long considered the titans of first-line therapy [4]. After 96 weeks time to virologic failure proved significantly faster in previously untreated people who started lopinavir/ritonavir (plus two nucleosides [NRTIs]) rather than efavirenz (plus two NRTIs) (P = 0.006). But among people who suffered virologic failure, dual-class resistance emerged more often during efavirenz failure than during lopinavir failure. And people randomized to the boosted PI gained significantly more CD4 cells through 96 weeks than did people taking efavirenz.
Sharon Riddler (University of Pittsburgh) and ACTG colleagues recruited 753 antiretroviral-naive people with a viral load over 2000 copies and any CD4 count. The open-label design randomized study participants to standard doses of efavirenz or lopinavir/ritonavir, or to 533/133 mg of lopinavir/ritonavir twice daily plus standard-dose efavirenz. Everyone randomized to efavirenz or lopinavir/ritonavir also took 3TC, and individual clinicians picked the second NRTI: AZT or extended-release d4T or tenofovir. People randomized to both efavirenz and lopinavir/ritonavir took no nucleosides. Study participants had relatively advanced disease, with viral loads above 100,000 copies in just more than half of each arm and a median baseline CD4 count of 182. The trial used the old soft-gel lopinavir/ritonavir capsule taken twice-a-day, rather than the new Kaletra tablets taken once-a-day because the study started before the new tablets were available.
The first of two primary objectives was time to virologic failure, defined as (1) failure to push the viral load down 1 log (10-fold) or rebound before week 32 or (2) failure to have a sub-200 load or a rebound after week 32. The median follow-up was 112 weeks. The time to virologic failure was significantly shorter in the lopinavir/r plus 2 nukes arm as compared to the efavirenz plus 2 nukes arm. Using these metrics, the proportion of patients without virologic failure at week 96 was 76% for efavirenz, 73% for efavirenz+lopinavir/r, and 67% for lopinavir/r (Table 1). In a statistical analysis with a significance threshold below 0.016, lopinavir/ritonavir proved significantly worse than efavirenz in time to virologic failure.
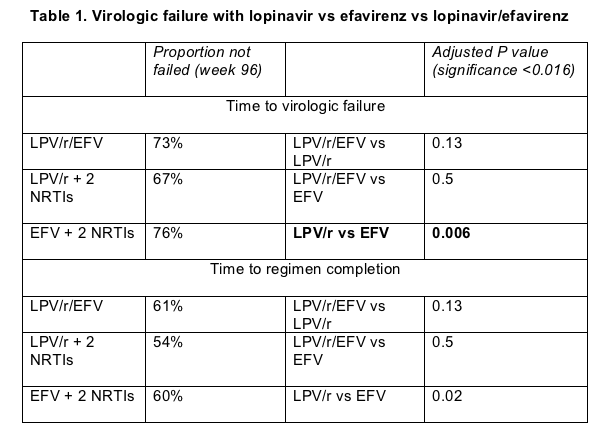
The second primary analysis measured time to regimen completion, defined as virologic failure or toxicity-related discontinuation of any drug in the regimen. For example, switching from AZT to d4T would constitute failure. This yardstick again showed a higher failure rate with lopinavir/ritonavir than with the efavirenz-containing regimens (Table 1), but the difference between arms fell short of statistical significance with a significance threshold set at P < 0.016.
In intention-to-treat analyses that ignored missing values, proportions with a week-96 viral load below 200 copies were 86% with lopinavir/ritonavir, 93% with efavirenz (P = 0.041), and 92% with both antiretrovirals. Proportions with week-96 loads under 50 copies came in at 77% for lopinavir/ritonavir, 89% with efavirenz (P = 0.003), and 83% with both drugs.
Preliminary analysis of resistance after virologic failure confirmed one result of other trials and yielded one surprise. As in the FIRST trial (also presented in Toronto and discussed below), failure of an NNRTI regimen opened the door to double-class resistance more often than PI failure did (Table 2). In the efavirenz arm 10 of 33 genotyped samples (30%) had virus resistant to two classes compared with 2 of 52 samples (4%) in the lopinavir/ritonavir arm and 2 of 39 samples (5%) in the lopinavir/ritonavir/efavirenz arm.
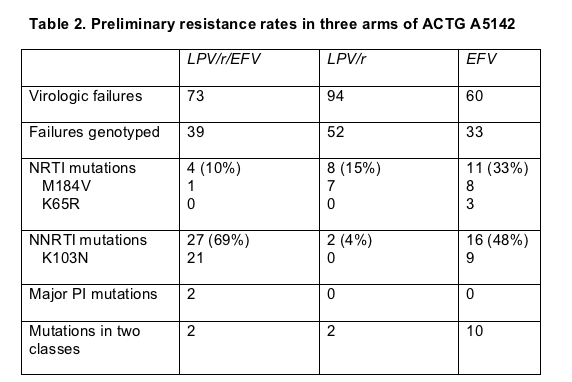
The surprise came with the high NNRTI resistance rate among people taking efavirenz with lopinavir/ritonavir--69% compared with 48% in the efavirenz-plus-NRTIs group. Riddler had no ready explanation for why two nucleosides appeared to stifle emergence of efavirenz-resistant virus better than lopinavir/ritonavir. Ultimately that finding may add to the list of reasons to avoid PI-NNRTI combinations in first-line therapy.
Another such reason is a higher risk of side effects when mixing a PI and an NNRTI than when combining a drug from those classes with nucleosides. While 33% taking lopinavir/ritonavir and 32% taking efavirenz had grade 3 or 4 lab abnormalities in this trial, 45% taking both drugs did. The greatest between-arm differences involved triglycerides above 750 mg/dL (14% with lopinavir/ritonavir/efavirenz versus 6% with lopinavir/ritonavir and 3% with efavirenz) and low-density lipoprotein cholesterol above 190 mg/dL) (6% with lopinavir/ritonavir/efavirenz versus 1% with lopinavir/ritonavir and 3% with efavirenz).
Both lopinavir-containing regimens pumped up CD4s significantly more than efavirenz plus two NRTIs, with median gains of 268 for lopinavir/ritonavir/efavirenz, 285 for lopinavir/ritonavir, and 241 for efavirenz (P = 0.01). But this statistical difference seems unlikely to have any short-term clinical import since Riddler reported no more clinical setbacks with efavirenz than with lopinavir-containing regimens.
Whether virologic results would have differed as much with the new lopinavir/ritonavir tablet, which may be taken once daily by people with no antiretroviral experience, is anyone's guess. The new tablet may be more tolerable than the old capsule, but tolerance differed hardly at all between the lopinavir/ritonavir arm and the efavirenz arm. For example, 19% in the lopinavir/ritonavir arm and 18% in the efavirenz arm reported any grade 3 or 4 clinical or lab "events." And treatment arms did not differ in time to first treatment-limiting toxicity. Riddler did not report adherence with the three regimens.
Baseline brackets have no impact on efavirenz response
Because early clinical research on nevirapine found a dimmed virologic response among people who started this nonnucleoside with a high viral load, doubts have persisted about whether NNRTIs deflate the highest loads as well as PIs. Impaired early responses among people with more advanced HIV infection have not cropped up in earlier efavirenz trials. But a fresh analysis of ACTG A5095 results should quell any lingering misgivings about efavirenz [5].
A5095, the much-parsed placebo-controlled trial that established the inferiority of three NRTIs (AZT, 3TC, and abacavir) to efavirenz-freighted first-line regimens [6] also showed that three NRTIs plus the nonnuke hogtie HIV no better than two NRTIs plus the nonnuke as a starting regimen [7]. The new analysis presented by Daniel Kuritzkes (Harvard Medical School, Boston) showed that--no matter how you slice pretreatment CD4 or RNA pie--efavirenz readily cuts HIV down to size.
After a median follow-up of 144 weeks in 765 people taking either efavirenz regimen, virologic response defined as a viral load kept below 200 copies did not differ much between those starting with fewer than 30,000 copies (29% of the study group), 30,000 to 99,999 copies (30%), 100,000 to 299,999 copies (17%), or more than 300,000 copies (24%). Nor did it matter if pretreatment CD4s stood below 50 (20% of the study group) or between 50 and 199 (27%), 200 and 349 (30%), 350 and 499 (14%), or over 500 (9%). An intent-to-treat analysis suggested a worse virologic response in the over-500 group, but Kuritzkes explained that result reflected a high dropout rate in 500-plus group because many of those people figured they didn't need treatment.
Whether ACTG statisticians used Kaplan-Meier analyses of time to virologic failure, Cox proportional hazard models of virologic failure risk, 144-week sub-50-copy rates, or CD4 change from baseline, a higher pretreatment viral load or lower pretreatment CD4 count did not jeopardize response to efavirenz plus two or three nucleosides. Of course it took people with sky-high loads longer to get them below RNA radar, but Kuritzkes reported no between-group response differences by 48 weeks.
Efavirenz nonadherence may pose greater risk for blacks
Despite the convincing evidence of efavirenz potency regardless of HIV disease stage (see preceding section), one factor, race, may weigh against a good response to this NNRTI--at least in people who adhere poorly to their regimen. That possibility rests on yet another analysis of ACTG A5095, this one presented by Bruce Schackman (Cornell University, New York) [8]. The finding that shaky adherence inflates the risk of virologic failure more in blacks than whites taking efavirenz could have global portent if it applies to any blacks taking NNRTIs, not just to the African Americans studied in ACTG A5095.
ACTG researchers had already reported that efavirenz regimens faltered in blacks more than whites and that blacks enrolled in this study had worse adherence than whites. The new analysis untangled the links between race, efavirenz failure, and adherence by measuring adherence in four ways:
- 4-Day recall: reporting not missing any doses of any active agent during the 4 days before the evaluation
- Weekend: reporting not missing any doses of any active agent during the weekend before the evaluation
- Last month: reporting not missing any doses of any active agent within the last month
- Never missed: reporting not missing any doses of any active agent during the 4 days before the evaluation AND reporting never skipping medications
Overall adherence proved better with the less stringent pill-taking meters, 4-day recall (84%) and last weekend (90%) and worse with the more stringent measures, last month (67%) and never missed (48%). Defining failure as a confirmed load above 200 copies, Schackman charted a higher failure rate in people with recent nonadherence than in those with recent good adherence by all four adherence tests (P < 0.02).
No surprise there, but the analysis also showed that efavirenz flopped in significantly higher proportions of nonadherent blacks than nonadherent whites. Among 32 whites nonadherent by 4-day recall, only 6 (19%) ended up in virologic failure. But among 50 blacks who reported poor adherence by 4-day recall, 23 (46%) had a virologic failure, a highly significant difference from nonadherent whites (P < 0.001). Eighteen whites scored themselves nonadherent on the weekend test, and efavirenz failed in 2 of them (11%), compared with 15 of 29 blacks (52%) reporting nonadherence on that test (P < 0.001).
Efavirenz failure comparisons by the other two adherence barometers also significantly favored whites. On top of that, time to virologic failure proved significantly faster when comparing nonadherent blacks with adherent blacks, but not when comparing nonadherent whites with adherent whites, according to the last-month test (P < 0.01), the never-missed test (P = 0.04), and the last-week test (P = 0.05).
Why poor adherence threatens nonadherent blacks so much more than nonadherent (or adherent) whites remains a mystery, but the ACTG team has some hypotheses. Answering an e-mail query, Cornell University's Roy Gulick, A5095's principal investigator, listed three possibilities that may merit future study: (1) differences in adherence patterns between blacks and others, for example, missing an occasional dose versus missing all doses for 1 week, (2) differences in low-level side effects causing differential toxicity and resulting in partial adherence, and (3) differences in social supports.
The worse virologic response in blacks versus whites with poor adherence is doubly surprising because other work suggests that a genetic difference between blacks and whites results in higher efavirenz levels among blacks [9] (though that may cause the possible race-based side effect difference that Gulick mentioned). Other recent research indicates that people can get away with wobbly adherence to NNRTIs more easily than subpar adherence to the unboosted PIs indinavir or nelfinavir [10,11]. A just-published study of 110 marginally housed people that measured adherence by unannounced pill counts found that most people taking efavirenz or nevirapine controlled viral replication with at least a 54% adherence rate, while it took 73% adherence for most people taking a PI to stifle HIV [11].
Analyzing these studies, A5095's chief, Roy Gulick, proposed that "sustained virologic suppression even with some degree of imperfect adherence" to NNRTIs probably reflects several factors, "including the inherent antiretroviral potency of NNRTI drugs (in combination with nucleoside analogues), as well as their convenience, tolerability, and long plasma drug half-lives" [12]. Gulick suggested this greater "forgiveness" with efavirenz or nevirapine probably also applies to ritonavir-boosted PIs.
Do blacks have more side effects with efavirenz?
The earlier ACTG study confirming higher efavirenz levels in blacks than whites also found more central nervous system (CNS) side effects 1 week after starting efavirenz in people with a genotype more common among blacks [9]. At the Toronto meeting analysis of a different trial disclosed no greater risk of CNS toxicity in blacks than in whites or Hispanics, though Hispanics suffered dizziness more than the other groups [13]. Virologic response to efavirenz did not differ significantly by race or ethnicity in this trial, which compared a switch to efavirenz with a continued twice-daily nonefavirenz regimen. A 48-week noncompleter-equals-failure analysis charted a statistically equivalent 77% sub-50-copy response rate among 66 blacks, an 82% response among 73 whites, and an 82% response among 60 Hispanics.
Looking at all grades of central nervous system side effects [see note 14], this US team found that 14 blacks (21%), 13 whites (18%), and 17 Hispanics (28%) had one or more such problems, though differences between groups were not statistically significant. Hispanics experienced dizziness significantly more often (20%) than blacks (6%) or whites (9%) (P < 0.05 versus whites). Considering only grade 2 to 4 side effects, these researchers again found statistically similar overall rates in blacks (8%), whites (8%), and Hispanics (13%), but significantly more dizziness in Hispanics (10%) than in blacks (0%) or whites (2%) (P < 0.05 versus whites).
NNRTIs outdo (mostly unboosted) PIs in FIRST
Results of the 5-year FIRST trial reflect ACTG A5142 in finding a better virologic response to first-line NNRTIs than PIs [15]. But most FIRST participants randomized to NNRTI therapy took efavirenz, while 74% randomized to PIs used no ritonavir boost.
The study found no difference between NNRTI and PI regimens in a composite endpoint including CD4 drop, progression to AIDS, and death--the study's primary endpoint. As in ACTG A5142, nonnuke failure resulted in more dual-class resistance than PI failure. And FIRST confirmed that triple-class therapy holds no virologic edge over two-class treatment for people trying their inaugural regimen, but triple-class combos did cause more side effects.
FIRST echoes results of two earlier trials that randomized previously untreated people to a PI, an NNRTI, or both--ACTG 384 [2,16] and INITIO [17]. Everyone in ACTG 384 and INITIO took either efavirenz, or nelfinavir, or both, whereas 61% in FIRST's PI arm took nelfinavir and 26% started a boosted PI. In FIRST's nonnucleoside arm, 63% took efavirenz and the rest nevirapine. With 1397 enrollees, FIRST was bigger than the other two trials, it boasted a longer median follow-up, and it recruited people with more advanced HIV infection (Table 3).
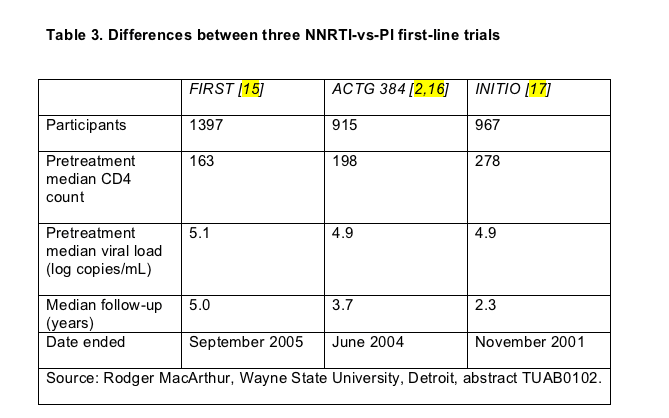
The percentage of FIRST participants who got their viral load under 50 copies and kept it there proved significantly lower in the PI group than in the NNRTI group or the triple-class group. Compared with people starting a PI regimen, those taking an NNRTI had a 1.63 times higher chance of staying below 50 copies (95% confidence interval 1.36 to 1.95). But that result must be interpreted in light of predominant nelfinavir use in the PI arm and predominant efavirenz use in the NNRTI arm. More people randomized to a PI than to an NNRTI switched their treatment strategy at least once (43.2% versus 31.7%), and median time to first switch was shorter in the PI group (18.2 versus 23.5 months).
After 5 years of follow-up the three treatment groups differed not at all in the composite primary endpoint of a sub-200 CD4 count, a new AIDS diagnosis, or death. A PI regimen did not differ from an NNRTI regimen in risk of death, AIDS, AIDS or death, a grade 4 "event," or stopping treatment because of toxicity. For reasons that remain unexplained, however, Hispanics had a higher death rate if taking a PI rather than an NNRTI (8.5 versus 5.6 per 100 person-years), while whites had a higher death rate with NNRTIs than PIs (5.6 versus 4.2 per 100 person-years). Risks of AIDS, death, a CD4 count under 200, a grade 4 toxicity, or stopping an antiretroviral because of toxicity were all marginally higher in the triple-class group than in the double-class groups.
Among people who suffered virologic failure in FIRST, rates of single-class resistance did not differ significantly with a PI (31.3%), an NNRTI (31.4%), or a treble-class combo (38.4%). But NNRTI failure bred multiclass resistance more often (22.3%) than did PI failure (13.8%) or triple-class failure (14.2%).
Efavirenz vs boosted PIs in a cohort study
FIRST's results can best be understood in the turn-of-the-century context of that trial's recruitment, when NNRTI-versus-PI trials [2,16,17] compared the two drugs most thought the best of class--efavirenz and nelfinavir--as FIRST itself largely did. Saquinavir/ritonavir had been studied (and used) for years when FIRST signed up its first participant, but it's easy to forget that lopinavir/ritonavir did not win approval until 2000. Seen from that side of the rabbit hole, FIRST's primary virologic finding amounts to little more than a confirmation of ACTG 384 [2,16] and INITIO [17]. So FIRST says little about how the more popular NNRTI, efavirenz, does against standard-of-care ritonavir-backed PIs (though that would be an interesting post hoc analysis).
But a cohort study detailed in Toronto's poster halls did referee a match between efavirenz and ritonavir-propped PIs, and efavirenz snagged the champion's belt, though odds seemed stacked in the nonnuke's favor [18]. As in ACTG A5142 [4], this 929-person study at Baltimore's Johns Hopkins and Nashville's Vanderbilt saw no AIDS-or-death difference between efavirenz and a boosted PI. But people whose first potent combo relied on efavirenz kept HIV in a hammerlock longer than PI therapy.
Vanderbilt contributed 398 cohort members and Hopkins 531, all of whom started either an efavirenz combination or a boosted PI (excluding boosted saquinavir) between June 1998 and April 2005. Because this is a cohort study and not a randomized trial, the efavirenz and PI groups differed considerably, starting with numbers--650 taking efavirenz and 279 a boosted PI. The efavirenz group included a bigger proportion of blacks (64% versus 55%, P = 0.02) and a bigger share of injecting drug users (31% versus 23%, P = 0.01).
People starting PIs had more advanced disease, with a median baseline CD4 count of 123 (versus 160 with efavirenz, P = 0.002) and a median baseline viral load of 72,391 copies (versus 50,100 copies with efavirenz, P = 0.003). So you might say the PI takers started this match with one arm tied behind their backs, or at least with a heavier viral hangover. Median treatment duration in this analysis stretched almost twice as long with efavirenz (69 weeks versus 36 weeks with PIs, P < 0.001), as did average follow-up time (104 versus 54 weeks, P < 0.001).
Defining durable suppression liberally as more viral loads below than above 400 copies in people with two or more viral load tests, Timothy Sterling and coworkers reckoned significantly more durable responses in the efavirenz contingent (52% versus 37%, P < 0.001). Despite that advantage, an analysis weighing several disease progression factors did not rank efavirenz as an independent predictor of slower progression. But in this multivariate analysis durable virologic suppression by itself halved the risk of progression (hazard ratio 0.50, P < 0.001). Several other progression predictors emerged at the following hazard ratios (HR):
- Pre-HAART CD4 count below 200 versus above 350: HR 2.25, P = 0.02
- Pre-HAART CD4% below 13%: HR 1.60, P = 0.01
- Injecting drug use: HR 1.61, P = 0.002
- Care at Hopkins versus Vanderbilt: HR 2.14, P < 0.001 (Hopkins has more injecting drug users.)
Two other predictors fell short of statistical significance:
- Pre-HAART CD4 count of 200 to 350 versus more than 350: HR 1.64, P = 0.17
- Pre-HAART viral load above 100,000 copies: HR 1.32, P = 0.08
Sterling and colleagues observed that the relatively short follow-up in their study (about 2 years with efavirenz and 1 year with a boosted PI) limits application of their findings to people taking antiretrovirals longer. And as FIRST showed, people on a failing first regimen typically switch fast to something else, and that limits the pileup of progression endpoints. The Hopkins-Vanderbilt team suggested the independent risk of progression with a CD4% under 13% may give clinicians another meter to gauge when a person should start antiretrovirals.
An already published Italian cohort comparison of 348 people starting efavirenz and 124 starting lopinavir/ritonavir in their first regimen yielded results similar to ACTG A5142--a significantly better virologic response to efavirenz, and a significantly better CD4 response to the PIs [19].
First-line bottom lines
Results of the NNRTI-versus-PI studies at the International AIDS Conference will spark no upheaval in antiretroviral prescribing. For the most part, the findings of ACTG A5142 [4], FIRST [15], and the Hopkins-Vanderbilt cohort study [18] confirm what everyone already knew:
- Efavirenz or a ritonavir-boosted PI makes a solid cornerstone for first-line therapy.
- Failure of an NNRTI regimen may take longer than failure of a boosted PI, but virologic failure of an NNRTI more often breeds double-class resistance.
- Risk of disease progression does not vary much in people who start with an NNRTI versus a boosted PI.
That last conclusion must be read in light of the Antiretroviral Therapy Cohort Collaboration finding that people whose first regimen hinges on a ritonavir-boosted PI do have about a 45% higher risk of progression to AIDS or death than people who start with efavirenz [3], though that finding comes with several weighty caveats reviewed above (see introductory paragraphs).
ACTG A5142 and FIRST had little to add on the other critical variable in first-line planning--short- and long-term toxicities. In preliminary analyses the ACTG team saw little difference between efavirenz and lopinavir/ritonavir in tolerance or lab readings (see "Efavirenz trumps lopinavir" above). Judith Shlay (Denver Public Health) offered a detailed analysis of lipid, glucose, and insulin meanderings in a substudy of 422 FIRST enrollees [20], but only one significant difference between the PI and NNRTI arms emerged. Reflecting earlier findings, people taking an NNRTI gained significantly more "good" high-density lipoprotein cholesterol than did people taking a PI (P < 0.05). Shlay saw no significant differences between the PI and NNRTI arms in triglycerides, low-density lipoprotein cholesterol, glucose, or insulin. But only 18% in the PI group of this analysis used a ritonavir boost.
Perhaps more than anything, the efavirenz-PI studies spotlighted in Toronto prove just how fast antiretrovirals come in and out of favor. FIRST's results show the patina of age because boosted PIs largely ousted their unboosted brethren in first-line combos as this trial's enrollment came to a close. Even ACTG A5142--fresh from the statisticians' counting house--looks a little dowdy because it used the older lopinavir/ritonavir formulation, and because many clinicians now prefer atazanavir if starting a boosted PI.
In the same way some new antiretroviral will eventually upstage efavirenz. Maybe the integrase inhibitor MK-0518. A randomized comparison of first-line efavirenz (plus tenofovir/3TC) with various doses of MK-0518 (plus tenofovir/3TC) showed that equivalent proportions taking higher doses of the integrase inhibitor or efavirenz had viral loads under 50 copies after 24 weeks [21]. But loads fell significantly faster in the MK-0518 arms, a finding that may mean this new drug packs a stronger antiviral punch.
Mark Mascolini writes about HIV infection (markmascolini@earthlink.net).
References and Notes
1. Staszewski S, Morales-Ramirez J, Tashima K, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med 1999;341:1865-1873.
2. Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med 2003;349:2293-2303.
3. The Antiretroviral Therapy Cohort Collaboration. Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis 2006;194:612-622.
4. Riddler SA, Haubrich R, DiRienzo G, et al. A prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV infection--ACTG 5142. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THLB0204.
5. Ribaudo H, Kuritzkes D, Lalama C, et al. Efavirenz-based regimens are potent in treatment-naive subjects across a wide range of pre-treatment HIV-1 RNA and CD4 cell counts: 3-year results from ACTG 5095 (A5095). XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THLB0211.
6. Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 2004;350:1850-1861.
7. Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA 2006;296:769-781.
8. Schackman B, Ribaudo H, Krambrink A, et al. Racial differences in self-reported adherence and association with virologic failure on efavirenz-containing regimens for initial HIV therapy: results from ACTG A5095. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract TUPE0113.
9. Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004;18:2391-2400.
10. Maggiolo F, Ravasio L, Ripamonti D, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis 2005;40:158-163.
11. Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis 2006;43:939-941 (http://www.journals.uchicago.edu/CID/journal/issues/v43n7/38663/38663.html).
12. Gulick RM. Adherence to antiretroviral therapy: how much is enough? Clin Infect Dis 2006;43:942-944 (http://www.journals.uchicago.edu/CID/journal/issues/v43n7/40286/40286.html).
13. Sension M, Jayaweera D, Lackey P, et al. Impact of race on efficacy and occurrence of nervous system symptoms in HIV-infected patients following simplification from a twice-daily or more frequent HAART regimen to a once-daily efavirenz-based HAART regimen. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THPE0141.
14. Central nervous system side effects included dizziness, insomnia, impaired concentration, somnolence, abnormal dreaming, euphoria, confusion, agitation, amnesia, hallucinations, stupor, abnormal thinking, and depersonalization.
15. MacArthur RD, Novak RM, Peng G, et al. Long-term clinical and immunologic outcomes are similar in HIV-infected persons randomized to NNRTI vs PI vs NNRTI + PI-based antiretroviral regimens as initial therapy: results of the CPCRA 058 FIRST study. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract TUAB0102.
16. Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med 2003;349:2304-2315.
17. Yeni P, Cooper DA, Aboulker JP, et al. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet 2006;368:287-298.
18. Sterling T, Barkanic G, Raffanti S, et al. Does optimal timing of HAART initiation differ with newer treatment regimens that include efavirenz or ritonavir-boosted protease inhibitors? XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract TUPE0205.
19. Torti C, Maggiolo F, Patroni A, et al. Exploratory analysis for the evaluation of lopinavir/ritonavir-versus efavirenz-based HAART regimens in antiretroviral-naive HIV-positive patients: results from the Italian MASTER Cohort. J Antimicrob Chemother 2005;56:190-195.
20. Shlay J, Bartsch G, Peng G, et al. Long-term changes in lipids and glucose/insulin levels among HIV-infected antiretroviral naive persons randomized to PI vs. NNRTI vs. PI + NNRTI-based antiretroviral regimens: results of the CPCRA 061 metabolic study. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THAB0101.
21. Markowitz M, Nguyen BY, Gotuzzo F, et al. Potent antiretroviral effect of MK-0518, a novel HIV-1 integrase inhibitor, as part of combination ART in treatment-naive HIV-1 infected patients. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THLB0214. (Mike Youle from London's Royal Free Hospital discusses MK-0518 and other investigational antiretrovirals in an article at this site [http://www.natap.org/2006/IAS/IAS_91.htm].)
|
| |
|
 |
 |
|
|