 |
 |
 |
| |
Darunavir Equally Effective After LPV, FPV, or TPV Failure
|
| |
| |
46th ICAAC, September 27-30, 2006, San Francisco
Mark Mascolini
September 29, 2006
Darunavir/ritonavir controlled viral replication in 40% of people taking a failing lopinavir, fosamprenavir, or tipranavir regimen regardless of which protease inhibitor (PI) was failing.
That finding emerged from a pooled analysis of 317 people in the POWER 1, 2, and 3 trials taking either lopinavir, fosamprenavir, or tipranavir in the regimen that was failing when they entered the POWER studies. Everyone in those trials had one or more major PI mutations and experience with at least one drug from the first three antiretroviral classes. Trial participants in this analysis took darunavir/ritonavir at the dose licensed for treatment-experienced people, 600/100 mg twice daily, plus two or more nucleosides and possibly enfuvirtide. Tibotec researchers did not report whether first-time enfuvirtide use differed in the three groups analyzed.
Statisticians calculated sub-50-copy responses by the FDA-favored time-to-loss-of-virologic-response algorithm. They figured change in viral load by a noncompleter-equals-failure analysis. After 24 weeks of darunavir therapy, the study group averaged a 1.74-log drop in viral load, and 42% had a load below 50 copies/mL. Virologic response differed hardly at all between the three groups studied (Table 1).
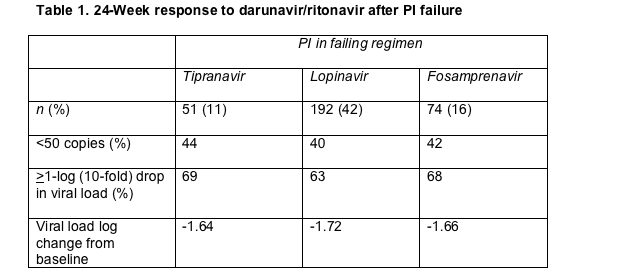
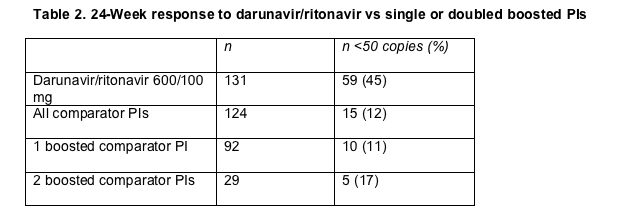
These response rates did not differ much from those in the entire POWER 1-3 population. The overall 24-week virologic response rate for people taking 600/100 mg of darunavir/ritonavir in the POWER studies was -1.74 log copies/mL, and 45% of people taking that dose in POWER 1 or 2 reached a load below 50 copies by week 24. People who took two ritonavir-boosted comparator PIs in these two trials did better than those who took a single boosted comparator PI, but sub-50-copy rates with double boosted PIs did not match the rate with darunavir/ritonavir (Table 2).
Reference
1. Lefebvre E, De Bethune M, De Meyer S, et al. Impact of use of TPV, LPV and (f)APV at screening on TMC114/4 virologic response in treatment-experienced patients in POWER 1, 2 and 3. 46th ICAAC. September 27-30, 2006, San Francisco. Abstract H-1387.
|
| |
|
 |
 |
|
|