 |
 |
 |
| |
Lower Reported Rate of Suspected Hypersensitivity Reactions (HSR)
to Abacavir (ABC) Among Black Patients
|
| |
| |
Reports by Jules Levin
ICAAC, Sept 27-30, 2006, San Francisco
Authors: C Brothers1, P Wannamaker1, D Sutherland-Phillips1 & J Hernandez1
1GlaxoSmithKline, Research Triangle Park, NC, USA
Brief Summary: GSK looked across a number of studies in which abacavir was used and found that Blacks had lower rates of reported abacavir-associated hypersensitivity reaction than Caucasians. It is believed that Blacks may be less likely to experience an abacavir-associated hypersensitivity reaction (HSR) and that this is due to a genetic difference. Further studies are ongoing to better understand and characterize this. GSK is in the process of studying a genetic test to identify who has the genetic marker for hypersensitivity. If the genetic test is successful we should be able to identify who is likely not to be at risk for developing a hepersensitivity reaction to abacavir.
ABSTRACT
Background: ABC is an effective drug for HIV-1 infection. Approximately 5% of patients treated with ABC develop HSR that in rare cases has proved fatal. An association between ABC HSR and carriage of the genetic allele HLAB* 5701 has been reported by several groups and reaches high statistical significance in Caucasian subjects but is less significant in Black subjects. In multivariable analyses assessing clinical risk factors for HSR, black race consistently demonstrated a lower odds for reporting HSR.
Methods: Reported rates of HSR from recent randomized, controlled clinical trials using ABC-containing products were reviewed across the study populations and by self-report of black race.
Results: Incidence rates from 5 studies comprising 2800 subjects from the Americas and EU are described.
Conclusion: Across 5 randomized controlled trials, there was a consistently low rate of HSR reported among Black subjects. This finding supports previous risk factor findings and recently reported HSR rates in the DART trial (CROI 2006). The prevalence of HLA-B*5701 differs among racial groups and is low in people of black race, which may partially explain this finding. Additional research is ongoing to more fully address the association of the allele and HSR across racially diverse populations.
AUTHOR DISCUSSION
_ A limitation of these analyses is diagnostic precision of clinically suspected HSR; diagnostic precision may be less accurate in subjects of racial groups with a low prevalence of HLA-B*5701.
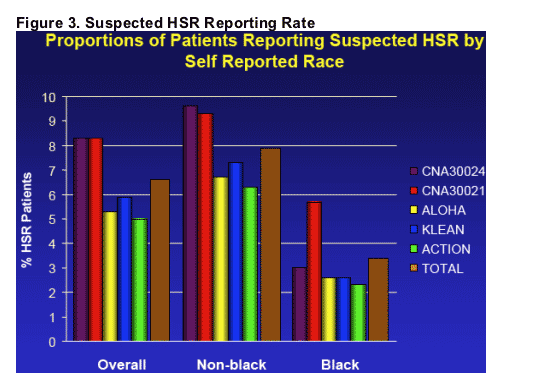
_ The overall reported rate of suspected HSR in these studies was 6.7% (range:5.5%-8.3%). There was a consistent difference observed in reporting rates between blacks (3.6% range: 2.3%-5.7%) and non-blacks (8.0% range: 6.3%-9.5%).
_ These data confirm and extend previous reports suggesting lower odds of HSR among patients of black race; additional research is needed and ongoing.
_ These findings do not suggest that clinical vigilance should be decreased in any patient initiating therapy with abacavir.
_ The prevalence of the HLA-B*5701 allele differs among racial groups and may partially explain this finding.
_ A large, prospective study is ongoing in Europe (PREDICT-1) to assess the clinical utility of screening patients for the HLA-B*5701 allele prior to therapy with abacavir.
_ A retrospective case-control study in African American patients with suspected ABC HSR compared to abacavir tolerant patients (SHAPE) is underway to better characterize this association in a non- Caucasian population.
AUTHOR CONCLUSIONS
_ Across 5 recent large, randomized controlled trials, there was a consistent difference noted in the reporting rates of suspected HSR among black and non-black subjects.
_ The lower reported rate of ABC HSR in subjects of black race in these 5 studies supports previous clinical trial data and risk factor findings.
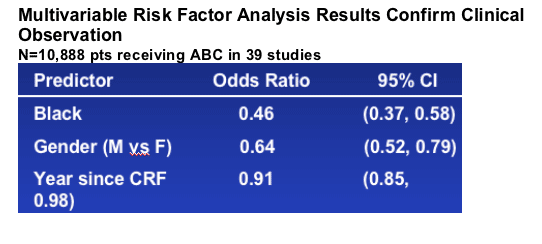
_ An updated risk factor analysis again found black race to a protective factor in reporting HSR.
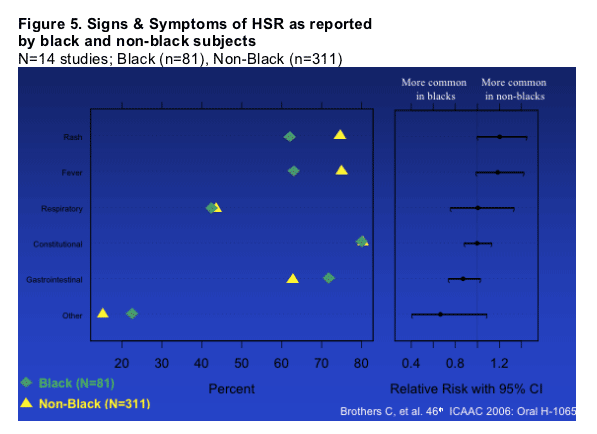
_ The clinical presentation (signs & symptoms) of HSR reported did not differ significantly between black and non-black subjects; there was a trend toward higher reporting of rash and fever in non-black subjects.
INTRODUCTION
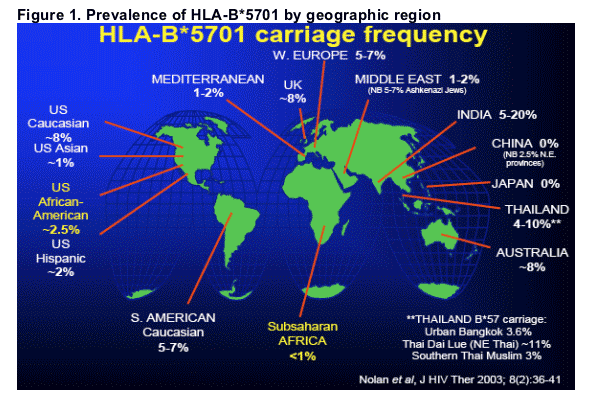
Abacavir (ABC) is effective and well tolerated in the majority of patients who begin therapy. The hallmark adverse event associated with ABC therapy is a hypersensitivity reaction which occurs in some patients. In multivariable clinical risk factor analyses (RFA), patients of black race have consistently been shown to have lower odds for developing HSR1. Recently, authors of the NORA substudy of the DART trial in Africa reported a low rate of HSR (2%) in their study2. An association between clinically suspected hypersensitivity and carriage of a particular genetic allele (HLA-B*5701) have been reported by several groups3,4. This association reaches high statistical significance among Caucasian subjects, but is less significant in subjects of black race5. The prevalence of this allele varies by geographic region and by race and is notably lower in people of African descent6,7 (Figure 1).
METHODS
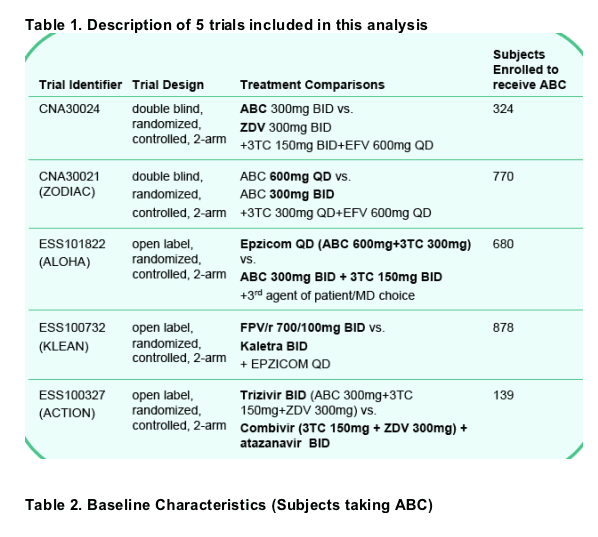
We describe the rate of suspected HSR reported among black patients compared to non-black patients across 5 recently reported, controlled clinical trials (Table 1).
Additionally, we characterized the clinical presentation (Signs & Symptoms) of HSR in black patients vs non-black patients according to details collected in a standardized data collection module (ABC HSR CRF) across 14 studies that used the collection module. Finally, an update to the previously reported Risk Factor Analysis1 was conducted.
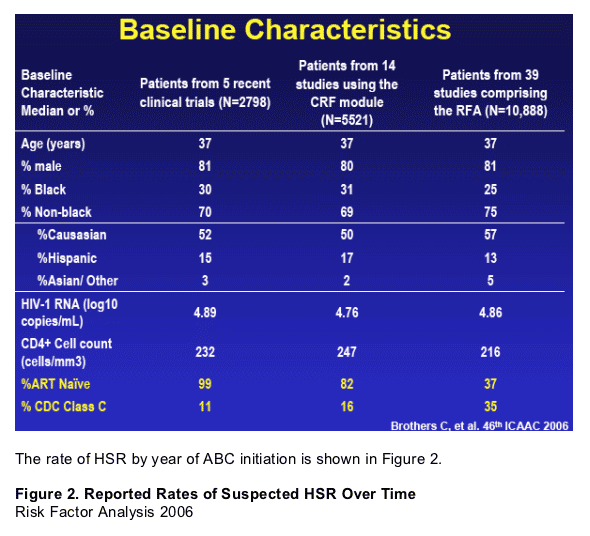
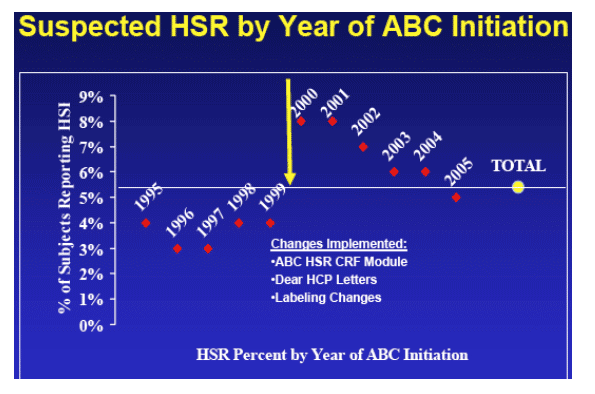
As seen in figure 2, the reported rates of HSR vary by protocol and over time. GSK implemented changes in the collection of HSR details in the year 2000 and also broadened the definition of HSR in professional labeling. The resulting spike in reporting rates is observed in Figure 2. The 14 studies in which the ABC HSR CRF module was used reported out in 2000 or later.
The reported rate of HSR across the 5 recent trials and by black race/non-black race is shown in Figure 3. The overall reported rate in these studies was 6.7% (5.5%-8.3%). There was a consistent difference observed in reporting rates between blacks (3.6% range: 2.3%-5.7%) and non-blacks (8.0% range: 6.3%-9.5%).
Updated multivariable risk factor analysis results confirm previous reports that black race is a protective factor in reporting HSR with an odds ratio of 0.46 (Figure 4). This analysis was updated to include results from two large studies (KLEAN and ALOHA) in which the majority of subjects received abacavir once daily as Epzicom/Kivexa.
Signs & Symptoms reported as part of the HSR syndrome were characterized from the 14 studies that used the targeted CRF Module (Figure 5). Although no differences reached statistical significance, trends were observed in proportions of subjects reporting fever and rash as part of the syndrome (less common among black subjects). And gastrointestinal more common in black subjects.
References
1. Cutrell et al. The Annals of Pharmacotherapy 2004
2. Munderi et al. CROI 2006
3. Mallal et al. Lancet 2002
4. Hetherington et al. Lancet 2002
5. Hughes et al. Pharmacogenomics 2004
6. Cao et al. Hum. Immunol. 2001
7. Nolan et al. J HIV Ther 2003
|
| |
|
 |
 |
|
|