 |
 |
 |
| |
Incidence of Drug Resistance Mutations in ART Naives from 2000 to 2005 in USA
|
| |
| |
Reported by Jules Levin
ICAAC, Sept 27-30, 2006, San Francisco
Brief Summary: HIV genotype data from 2035 antiretroviral-naive U.S. subjects located in 33 states (plus the District of Columbia) were obtained between 2000-2005 enrolled in GlaxoSmithKline clinical studies.
The percentage of ART-naive subjects with 'any' detected primary HIV drug mutations found in this study increased from about 8% in 2003 to 13-14% in 2004 to 16-17% in 2005. For detected NRTI HIV drug mutations the percent was about 3-4% in 2003, 4-6% in 2004, and 7-8% in 2005. For NNRTI HIV drug mutations the rate was 6% for the entire 2000-2005 period; the rate in 2000 was 2%, 6% in 2001, 6% in 2002, 5-6% in 2003, 7% in 2004, with a big increase in 2005 to 10-11%. So, from 2003 to 2005 the rate of NNRTI resistance doubled. The percent of PI mutations was 2% in 2001, 5% in 2002, 1-2% in 2003, 3% in 2004, and 4-5% in 2005.
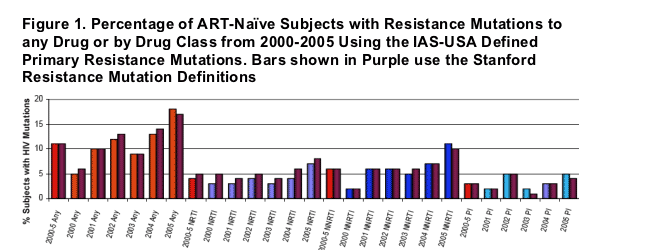
Figure 3. Overall Yearly Incidence of Primary Resistance Mutations in Specific Geographic Regions using either IAS-USA (Blue Bars) or Stanford Definitions (Purple Bars)
In this graph you can see the large increases in HIV drug primary resistance mutations particularly in the Western region of the USA from 2004 to 2005, where rates increased from 6-8% in 2004 to 25-26% in 2005. Rates in the Midwest also increased from 2004 to 2005 quite a bit. Rates went down in the Northeast during that time, while in the South rates were stable. From 2000 to 2005 rates in the Northeast were stable but increased quite a lot in all other regions, where they at least tripled.
AUTHOR DISCUSSION
The DHHS recently revised the "Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents" 1 to include a recommendation for genotypic resistance testing prior to initiation of antiretroviral therapy in patients with acute or chronic HIV infection.
The level of resistance cited (16%) was similar to the incidence observed in our cohort in 2005 (17-18%) when calculating resistance using either the Stanford database list or primary IAS-USA mutations. In this study, during 2005, this level primarily results from increased incidence of NNRTI mutations, which were observed in 10-11% of the subjects. Several other recent presentations2-4 have also noted increasing incidences of NNRTI resistance or resistance-associated mutations in ART-naive individuals, with NNRTI mutations or resistance being more frequently observed than either NRTI or PI mutations or drug resistance.
For many reasons, genotypic resistance testing may not be possible for every HIV-infected individual. However, given that transmission of NNRTI mutations appears to be rising, and that NNRTI resistance mutations tend to confer broad cross resistance among the NNRTI class, use of baseline resistance testing should be carefully considered if an NNRTI is to be used as part of the initial regimen. These results suggest that if resistance testing is not an option, then use of an NNRTI-sparing first line regimen should be considered, especially in areas such as New York, where a 13.4% incidence of transmitted NNRTI resistance has been reported for 2003-2004.5
AUTHOR CONCLUSIONS
Primary IAS-defined resistance mutations or the Stanford database mutation list for surveillance of primary HIV-1 resistance gave similar results.
Using either list, HIV resistance-associated mutations, especially NNRTI resistance mutations, increased significantly in US ART naive subjects from 2000-2005.
Transfusion risk was significantly associated with NNRTI resistance mutations, possibly reflecting reduced viral adaptation needs or greater initial diversity via this route. (note: several individuals who I was viewing the poster with thought this finding was strange & suggested perhaps individuals were not honest about risk factors since transfusion risk is old).
Black patients had significantly fewer PI and NRTI resistance mutations.
For subjects with HIV RNA ≦100,000 copies/mL, HIV resistance-associated
mutations increased significantly between 2000-2005.
POSTER
"Factors Associated with HIV-1 Mutations Linked to Drug Resistance in Antiretroviral Therapy (ART) Naive HIV-infected Individuals in the United States (US) from 2000-2005 (PREPARE Study)"
L. Ross1, A. Florance1, B. Wine1, D. Irlbeck1, C. Craig2, C. Vavro1, D. McClernon1, M. Tisdale2, R. Balu1, T. Lancaster1, K. Pappa1, and R. Lanier1
1GlaxoSmithKline, RTP, NC; 2GlaxoSmithKline, Stevenage, UK
INTRODUCTION
Virologic failure on ART is often linked to the presence of primary HIV-1 resistance associated mutations. In this study, we examined the potential association of several factors with detection of primary resistance associated mutations in ART-naive, HIV-infected subjects.
METHODS
Plasma-derived HIV was genotyped from HIV-infected, ART-naive subjects in the United States enrolling into GlaxoSmithKline clinical trials from 2000 through 2005.
Resistance-associated mutations were analyzed by two definitions:
1. As per the October 2005 IAS Drug Resistance Mutations Group (http://www.iasusa.org). Secondary resistance mutations as per this definition were not included. Primary mutations by class that were included are:
- NRTI: M41L, A62V, K65R, D67N, T69 insertions, K70R, L74V, V75I, V77L, Y115F, F116Y, Q151M, M184I/V, L210W, T215Y/F, K219Q/E.
- NNRTI: L100I, K103N, V106A/M, V108I, Y181I/C, Y188C/L/H, G190A/S, P225H, M230L, P236L
- PI: D30N, V32I, L33F, M46I/L, I47V/A, G48V, I50V/L, V82A/F/S/T/L, I84V, N88S, L90M
2. As per the Surveillance mutations list on the Stanford HIV Drug Resistance
website
(http://hivdb6.stanford.edu/asi/deployed/Surveillance/resistanceEstimator.cgi)
- NRTI: M41L, K65R, D67any, T69insertion, T69D/N, K70R, L74V, V75A/M/S/T,
V77L, Y115F, F116Y, Q151M, M184I/V, L210W, T215C/D/E/F/I/N/S/V/Y, K219E/Q/R
- NNRTI: L100I, 101E, K103N/S, V106A/M, Y181C/I, Y188C/H/L, G190A/E/Q/S,
P225H, M230L, P236L
- PI: L24I, D30N, V32I, M46I, I47A/V, G48V, I50L/V, F53L, I54A/L/M/S/T/V,
G73A/C/S/T, V82A/F/M/S/T, I84A/C/V, N88D/S, L90M
Changes over time in mutation rates were analyzed by drug class, geographic region, race and viral load. Geographic regions within the US were as defined by the US Census bureau. The overall incidences and incidence by year are presented. As few samples had protease genotyping from the year 2000, no PI mutation data are presented for that year. Data from any category with ≦20 subjects are not presented.
Logistic regression methodology was used to assess the relationship between the presence of IAS resistance mutations (any, NRTI, NNRTI and PI in separate models) and potential covariates or factors. The response or dependent variables (mutations) were classified as either present or not. The probability of mutation(s) being present was modeled. The following categorical covariates or factors that were investigated in the univariate logistic regression models for each of the 4 response variables were Risk Factors: Hemophilia; Homosexual; Injectable Drug; Occupational; Heterosexual; Transfusion; Vertical Transmission; Other risk factors; Gender; Race (combines "Other" with "White", "White" as reference); Region ("South" as reference); RNA Category (RNAC, 1=≦100K, 2=>100K). Continuous variables included: Age (years); CD4 (cells mm3); RNA (log10 copies/mL); Year (linear, 0-5 for 2000-2005).
All significant covariates from separate univariate models were then assessed together in the same model for each response variable in a multivariate logistic regression model using stepwise selection (entry into model set at 0.4). Where there were significant interactions, separate multivariate logistic models were also run for each categorical interaction covariate subgroup for purposes of interpretation.
RESULTS
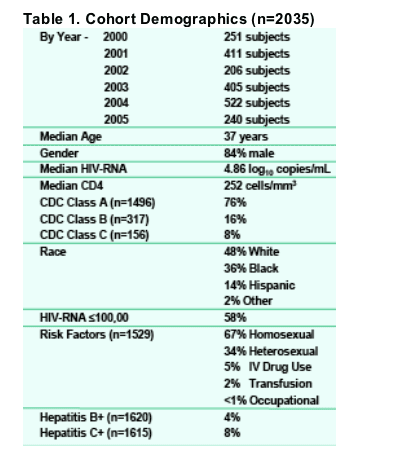
HIV genotype data from 2035 antiretroviral-naive U.S. subjects located in 33 states (plus the District of Columbia) were obtained between 2000-2005. Demographic data for the entire cohort is shown in Table 1. The incidence of resistance associated mutations was calculated using two different definitions (the primary IAS-USA mutation list and the Stanford database posted surveillance mutations list noted in the Methods section).
The incidence of any resistance associated mutations from all 3 drug classes (as one group) for the entire cohort from 2000-2005 was 11% using either the IAS-USA defined primary mutations list or using the Stanford mutation list.
Between 2000-2005, when analyzed by drug class, the NRTI resistance mutation incidence was 4%, NNRTI was 6%, and PI was 3% by the IAS-defined list, and 5%, 6% and 3%, respectively, using the Stanford list. Overall the incidence tended to increase over time, with specific yearly incidence shown in Figure 1. A significant increase in IAS-defined NNRTI resistance-associated mutations (OR 1.213, p=0.0011) as well as in the combined incidence of IAS defined primary resistance associated mutations from all three drugs classes (OR 1.209, p<0.0001) was seen from 2000-2005.
In the multivariate models the likelihood of subjects having any resistance mutations significantly increased over time between 2000-2005, (p=0.0151 IAS or p=0.0219 Stanford respectively). The likelihood of having either NRTI or NNRTI mutations significantly increased over time in either IAS (p=0.0419, p=0.0109 respectively) or Stanford (p=0.0116, p=0.0074 respectively).
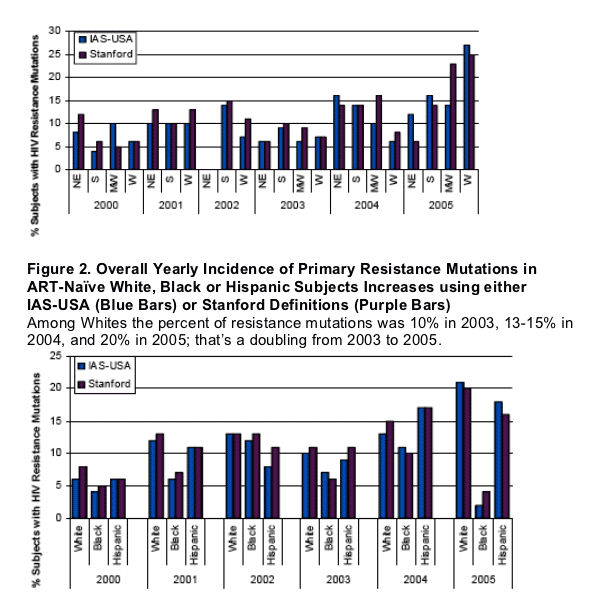
By race, the overall incidence of any resistance mutation from 2000-2005 was 12% for Whites, 12% in Hispanics, and 8% for Blacks per IAS defined primary mutations list or 13%, 12% and 6%, respectively, using the Stanford mutation definitions.
As seen in Table 2, significantly fewer Black subjects than Whites had primary IAS or Stanford defined mutations, or NRTI resistance mutations.
Black subjects had significantly fewer primary IAS defined PI mutations and significantly fewer Stanford defined NNRTI mutations. Figure 2 presents the yearly incidence of any resistance mutation by race. In general, the overall level of detected resistance increased for all race categories over time using resistance mutations as defined by IAS-USA or per Stanford definition.
In the multivariate models, Black subjects were significantly less likely than White subjects to have any mutation by IAS (p=0.0033) or Stanford (0.0012) definitions, significantly less likely to have any NRTI by IAS (p=0.0347) or Stanford (0.0157), any NNRTI mutation by Stanford definition (p=0.0042), and any PI mutation by IAS (p=0.0014).
Detection of resistance-associated mutations to a single drug class accounted for most of the resistance observed in these subjects. Between 2000-2005 the median incidence of ≥2-class resistance was 1% (range:<1-2%) using either primary IAS-USA or Stanford definitions of resistance mutations. When analyzed by geographic region or by racial group, the combined incidence of ≥2-class resistance from 2000-2005 also did not exceed 2% by either definition.
From 2000 through 2005, the incidence of IAS defined resistance mutations in subjects with HIVRNA ≦100,000 copies/mL was 10% and in subjects with HIV-RNA >100,000 copies/mL was 12%. In a univariate categorical analysis (Table 2), subjects with HIV-RNA ≦100,000 copies/mL were slightly less likely (OR 0.652, p=0.0326) to have NRTI mutations using the Stanford resistance mutation definitions.
When examining HIV-RNA as a continuous variable (Table 2), subjects with higher HIV RNA were significantly less likely to have IAS defined primary PI resistance mutations (OR 0.612, p=0.0164) and more likely to have NRTI resistance associated mutations (either IAS or Stanford definitions).
In a multivariate analysis of year and HIV-RNA interactions, subjects with HIV RNA <100K, were significantly more likely in later years to have greater numbers of IAS-defined resistance mutations (OR 1.259 p=0.0009) or Stanford defined resistance mutations (OR 1.192 p=0.0089). Subjects with HIV RNA <100K, were significantly more likely in later years to have greater numbers of NNRTI IAS-defined resistance mutations (OR 1.204 p=0.0364) or Stanford defined resistance mutations (OR 1.227 p=0.0246). Subjects with HIV RNA <100K, were significantly more likely in later years to have greater numbers of NRTI IAS-defined resistance mutations (OR 1.275 p=0.0487).
Although relatively few (2%) of the 2035 subjects in this cohort listed transfusion as a risk factor for having acquired HIV infection, as seen in Table 2, transfusion risk was associated with an increased probability (O.R.> 3) of selecting for NNRTI mutations by either IAS or Stanford definitions.
By geographic region, the overall resistance incidence between 2000-2005 using the IAS-USA and Stanford definitions, respectively were: 9% and 10% for the Northeast, 11% and 12% for the South, 9% and 12% for the Midwest, and 12% and 12% for the West. Figure 3 shows the incidence by year (groups with ≦20 subjects not shown). Over time, the numbers of subjects with resistance mutations detected appears to rise for all of the geographic regions using either resistance definition. Significantly more subjects in the West had NRTI mutations than in the South (Table 2).
REFERENCES
1. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. May 4, 2006 http://AIDSinfo.nih.gov
2. Little SJ et al Antiviral Therapy 2006 11 S110.
3. Smith D. et al Antiviral Therapy 2006 11 S121.
4. Eschelman SH et al Antiviral Therapy 2006 11 S122.
5. Shet A et al JAIDS 2006 41:439-446.
|
| |
|
 |
 |
|
|