 |
 |
 |
| |
Atazanavir/ritonavir (ATV/RTV) and Efavirenz (EFV) NRTI-Sparing Regimens
in Treatment-Naive Adults
|
| |
| |
Reported by Jules Levin
46th ICAAC, San Francisco, California, September 27-30, 2006
BMS-121: 48-Week Results
Douglas Ward, Larry Bush, Alexandra Thiry, Tong Guo, David Parks,
Joseph Jemsek, Thomas Jefferson, and Gary Thal for the -121 Study Group
Brief Summary:
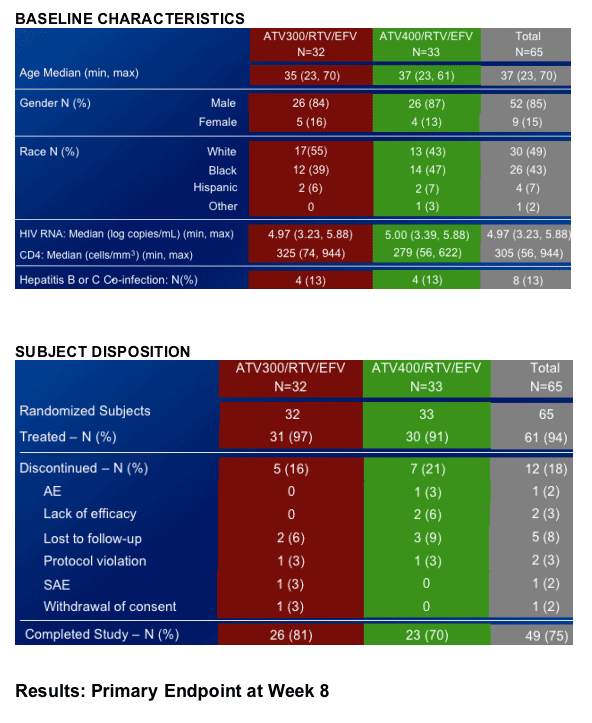
65 naive patients were randomized to either ATV/RTV 300/100 +EFV or ATV/RTV 400/100 + EFV; efavirenz lowers atazanavir levels.
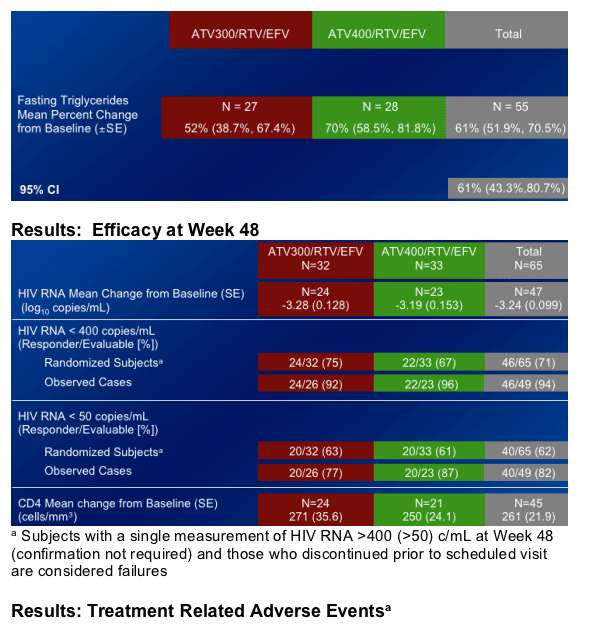
HIV RNA <400: 75% in ATV 300/r +EFV; 67% in ATV 400/r +RFV
HIV RNA <50: 63% in ATV 300/r +EFV; 61% in ATV 400/r +EFV
Effect on Lipids:
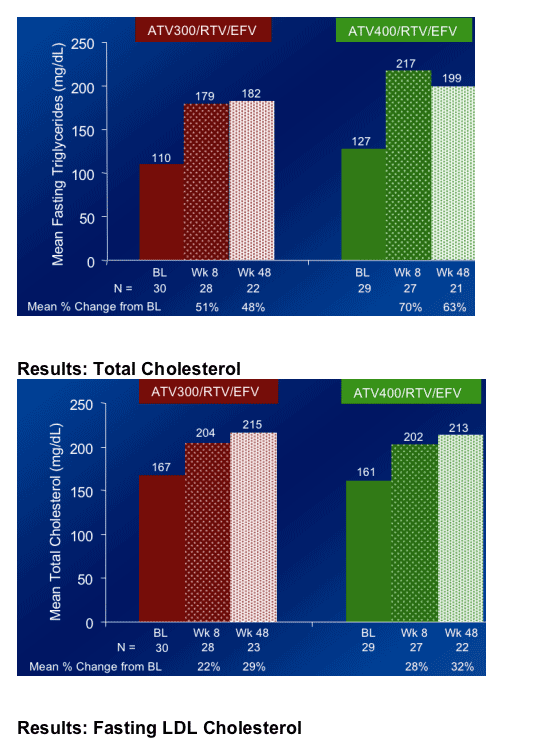
Fasting Triglycerides increased by 51% at week 8 from baseline with ATV300/r+EFV (this was Primary Study Endpoint), and by 70% at week 8 with ATV400/r+EFV
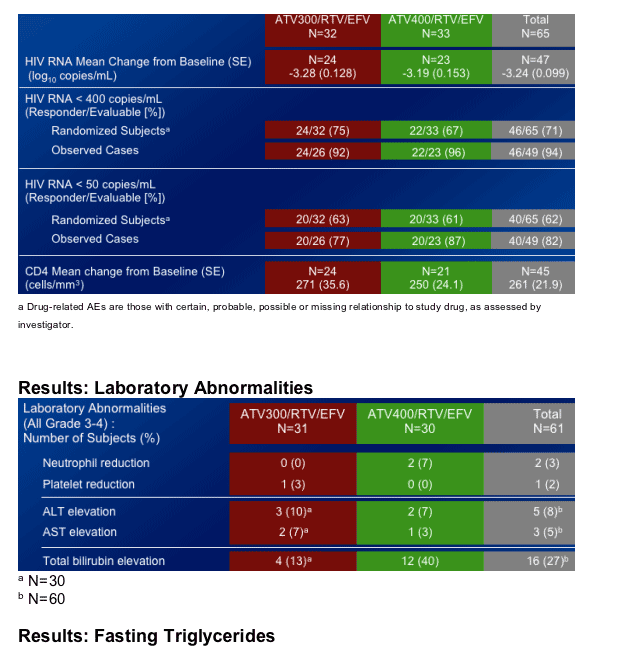
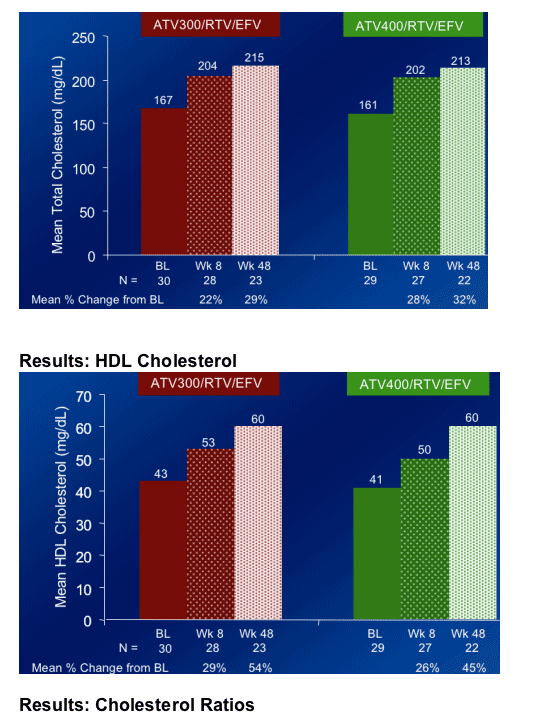
Total cholesterol increased by 22% & by 28% at week 8 in the ATV/300/r+EFV & ATV400/r+EFV arms, respectively.
Fasting LDL Cholesterol increased by 11% & 13% at week 8 in the ATV300/r+EFV & ATV400/r+EFV arms, respectively.
HDL Cholesterol increased by 29% & 26% at week 8 in the ATV300/r+EFV & ATV400/r+EFV arms, respectively.
The obvious lipid elevations associated with using this regimen do not compare with the favorable effect of atazanavir/r on lipids.
AUTHOR SUMMARY
Both ATV/RTV/EFV regimens demonstrated potent antiretroviral efficacy.
Both ATV/RTV/EFV regimens were generally safe and well tolerated through 48 weeks of treatment.
- The incidence of Grade 3-4 elevations in bilirubin was higher in the ATV 400/RTV/EFV arm.
There were no discontinuations due to bilirubin in either regimen.
Elevations in fasting plasma triglycerides were greater than those seen in previous studies of ATV, ATV/RTV or EFV at Week 48.
The mean percent change from baseline in fasting plasma triglycerides at Week 48 was 55% in the combined treatment regimens.
The incidence of Grade 2-4 elevations in triglycerides (>400mg/dL) at Week 48 was 5%, all Grade 2.
Changes in total, fasting LDL, and non-HDL cholesterol were more moderate in both regimens than the change in fasting triglycerides.
Elevations in HDL cholesterol in both ATV/RTV/EFV regimens were greater than seen in previous studies of ATV or EFV.
-- HDL increased 45%-54%
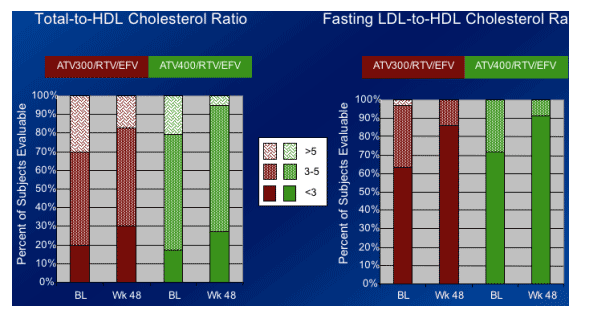
-- More favorable ratios of total:HDL and fasting LDL:HDL cholesterol were seen at Week 48 compared to baseline.
The relative impact of elevations in both HDL cholesterol and fasting triglycerides is unknown, and may warrant further investigation.
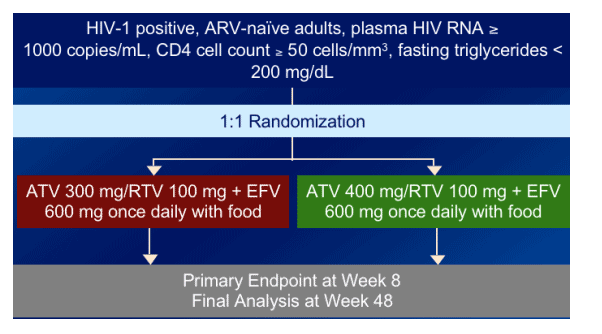
STUDY DESIGN
Naive patients with >50 CD4s & <200 triglycerides were randomized to ATV/RTV 300/100 + 600 mg efavirenz once daily with food or to ATV/RTV 400/100 + EFV once daily with food.
STUDY OBJECTIVES: Lipids
Primary Objective
To determine the mean percent change from baseline in fasting plasma triglycerides at Week 8 in the combined treatment regimens
Secondary Objectives
To evaluate in each treatment regimen
-- Mean percent change from baseline in fasting plasma triglyceride
--Incidence of Grade 2-4 elevations of fasting plasma triglycerides (in each treatment regimen and combined treatment regimens)
-- Change from baseline in fasting total, LDL, HDL and non-HDL cholesterol
-- Percentage of subjects requiring lipid lowering therapy
Study Objectives: Efficacy and Safety
Secondary Objectives
To evaluate in each treatment regimen
-- Magnitude and durability of the reduction of HIV RNA from baseline
-- Percent of subjects with plasma HIV RNA <400 copies/mL and <50 copies/mL
-- Change from baseline in CD4 cell count
-- Percent of subjects discontinuing due to jaundice and/or scleral icterus
General safety and tolerability
|
| |
|
 |
 |
|
|