 |
 |
 |
| |
U.S. Resistance & NNRTI Rates Still Rising in Untreated
|
| |
| |
Excerpted from Resistance Before Therapy, Hidden Mutations, and Blips
46th ICAAC, September 27-30, 2006, San Francisco
Mark Mascolini
US resistance rates still rising in untreated
Infection with resistant HIV, especially virus resistant to nonnucleosides (NNRTIs), continues to rise across the United States, according to results of a 2035-person study by Lisa Ross of GlaxoSmithKline [1]. Because of climbing NNRTI resistance in untreated people, Ross recommended first-line protease inhibitor (PI) therapy in regions with particularly high NNRTI resistance rates--unless a person's virus can be tested for resistance mutations before treatment.
The Glaxo study involved 2035 antiretroviral-naive people who enrolled in clinical trials from 2000 to 2005 in 33 states and Washington, DC. Most cohort members, 84%, were men, and more than 90% became infected during sex. Median viral load and CD4 count when people enrolled in the trials were 4.86 log copies/mL and 252 cells.
Figuring mutation rates according to the IAS-USA list of primary mutations and the Stanford database, Ross found little difference between the systems. From 2000 through 2005, prevalence of infection with resistant virus stood at 11% according to both the IAS-USA and Stanford schemes. NNRTI mutations proved most common by the IAS-USA system (6%), followed by nucleoside (NRTI) mutations (4%), and PI mutations (3%).
Prevalence of any pretreatment mutation rose from about 5% in 2000, to 10% in 2001, to a peak above 15% in 2005. From 2000 to 2005 the risk of any IAS-USA mutation climbed more than 20% (odds ratio [OR] 1.209, P < 0.0001) and the risk of any NNRTI mutation surged 21% (OR 1.213, P = 0.0011).
Over the 5 study years the risk of any IAS-USA resistance mutation jumped 12% for whites, 12% for Hispanics, and 8% for blacks. Multivariate analysis determined that, compared with whites, blacks had a significantly lower chance of infection with resistant virus from any class according to either IAS-USA (P = 0.0033) or Stanford definitions (P = 0.0012). The same held true for each drug class figured separately. The Glaxo team did not speculate on reasons for this discrepancy. Perhaps the most likely explanation is that fewer African Americans enrolling in these trials got infected by people taking antiretrovirals.
Among people with a pretreatment viral load below 100,000 copies, multivariate analysis figured an ever-escalating risk of infection with resistant virus from 2000 to 2005. Over that period people ran a 26% greater risk of becoming infected with a higher number of IAS-USA mutations (OR 1.259, P = 0.0009) and a 19% greater risk of infection with more Stanford-defined mutations (OR 1.192, P = 0.0089). These significantly higher risks in later years held true in separate analyses of NNRTI mutations and NRTI mutations, but not PI mutations.
Ross and colleagues concluded that the high primary resistance rates they recorded support US Department of Health and Human Services recommendations to genotype virus before treating people with acute or chronic HIV infection. The heightened risk of infection with NNRTI-resistant virus, confirmed in other recent studies,[2-4] led Ross to suggest that "if resistance testing is not an option, then use of an NNRTI-sparing first-line regimen should be considered, especially in areas such as New York, where a 13.4% incidence of transmitted NNRTI resistance has been reported for 2003-2004 [5]."
In a separate analysis of the Monogram resistance database, Ross found that 33.6% of viral isolates with no primary NRTI mutations had decreased susceptibility to one or more NNRTIs [6]. Because the 26,852 isolates collected from 2003 through 2005 were not linked to patient records, Ross could not tell how many of the NNRTI-resistant viruses came from treated versus untreated people. But recent work showing NNRTI resistance prevalence of 7% to 13% in untreated people [2-5] suggests that the larger portion of NNRTI-resistant isolates in the current study came from treated people.
Among isolates with one primary NRTI mutation, 53.8% had reduced susceptibility to an NNRTI, and among those with two primary NRTI mutations, 66.6% had resistance to an NNRTI. Rates of resistance to PIs were much lower in isolates with 0, 1, or 2 primary NRTI mutations (Table 1).
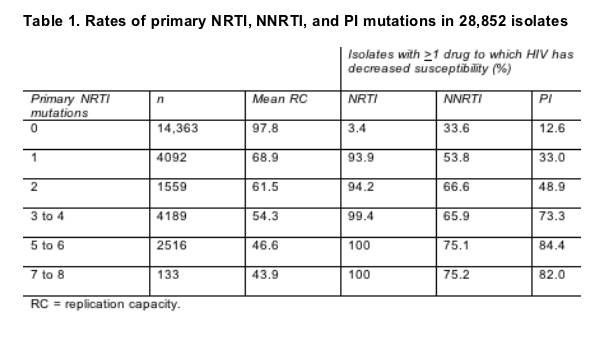
This study also traced a plummet in viral replication capacity after a single NRTI mutation evolves (Table 1), perhaps, Ross speculated, because M184V/I and K65R evolve early in failure and because either may compromise replication capacity. As additional NRTI mutations arose, replication capacity continued to drop, but more slowly than after emergence of the first NRTI mutation.
References
1. Ross L, Florance A, Wine B, et al. Factors associated with HIV-1 mutations to drug resistance in antiretroviral therapy naive HIV-infected individuals in the United States from 2000-2005 (PREPARE Study). 46th ICAAC. September 27-30, 2006. San Francisco. Abstract A-378.
2. Little SJ, May S, Hecht F, et al. Increase in transmitted NNRTI drug resistance among recently HIV-infected patients from North America and Australia. Antiviral Ther 2006;11:S110.
3. Smith D, Pesano R, Cachay E, et al. Prevalence of HIV drug resistance among antiretroviral naive individuals of unknown infection duration. Antiviral Ther 2006;11:S121
4. Eshelman SH, Husnik M, Hudelson S, et al. Analysis of antiretroviral drug resistance and HIV-1 subtype among men who have sex with men recently infected with HIV-1 in the United States: the EXPLORE Study. Antiviral Ther 2006;11:S122.
5. Shet A, Berry L, Mohri H, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. JAIDS 2006;41:439-446.
6. Ross L, Parkin N, Lanier R. The number of HIV primary NRTI mutations correlates directly with other antiretroviral associated mutations and indirectly with replicative capacity and reduced drug susceptibility. 46th ICAAC. September 27-30, 2006. San Francisco. Abstract H-1001.
|
| |
|
 |
 |
|
|