 |
 |
 |
| |
Safety/Lipids & Durability of Viral Load Suppression of TMC114 in POWER 1/2 Week 48
|
| |
| |
Reported by Jules Levin
"Durability of Viral Load Suppression with Darunavir/ritonavir in Treatment-experienced Patients: POWER 1 and 2 Combined Week 48 Analysis"
poster 958, IDSA Conference, Toronto, Oct 12-15, 2006
Nicholaos C. Bellos, MD1; Ron Falcon, MD2; Andrew Hill, PhD3
1Dallas, TX; 2Tibotec Therapeutics, Bridgewater, NJ, USA; 3University of Liverpool, Liverpool, UK
ABSTRACT
Background: In the treatment of adults and adolescents with HIV, achieving HIV RNA <50 copies/mL is a key treatment goal as outlined by the DHHS guidelines. Accordingly, the standard primary efficacy endpoint in clinical trials of antiretroviral (ARV) drugs in treatment-na´ve HIV patients (pts) is HIV RNA <50 copies/mL at Week (Wk) 48. In treatment-experienced pts, it has traditionally been more difficult to achieve undetectable RNA, leading to log10 reduction in HIV RNA at Wk 24 as the primary endpoint in recent pivotal trials of ARV drugs studied in this population. POWER 1 and 2 evaluated the efficacy of darunavir (DRV; formerly TMC114) with low-dose ritonavir (DRV/r) in treatment-experienced pts with HIV.
Methods: Durability of ≥1 log10 reduction in HIV RNA and <50 copies/mL endpoints were analyzed at Wks 24 and 48. Analyses were conducted in pts receiving the DRV/r 600/100mg bid dose. All analyses were intent-to-treat using time-to-loss of virologic response.
Results: The overall percentage of pts with ≥1 log10 reduction in HIV RNA from baseline was 70% at Wk 24 and 61% at Wk 48, while the percentage of pts achieving HIV RNA <50 copies/mL remained similar between Wk 24 (45%) and Wk 48 (45%). HIV RNA <50 copies/mL was the most durable endpoint: 90% of pts reaching <50 copies/mL at Wk 24 sustained this response to Wk 48, compared with 70% of pts who sustained a ≥1 log10 reduction in HIV RNA from Wk 24 to Wk 48.
Conclusions: HIV RNA <50 copies/mL is an achievable goal for treatment-experienced patients and is more durable than an endpoint of ≥1 log10 reduction in HIV RNA. Achieving HIV RNA <50 copies/mL may be useful as a primary efficacy endpoint for trials in all patient populations.
Safety
DRV/r was generally well tolerated; the majority of adverse events were mostly mild to moderate (Grade 1 to 2) in severity.
Discontinuations due to adverse events were infrequent: 9% for DRV/r and 5% for control PI(s).
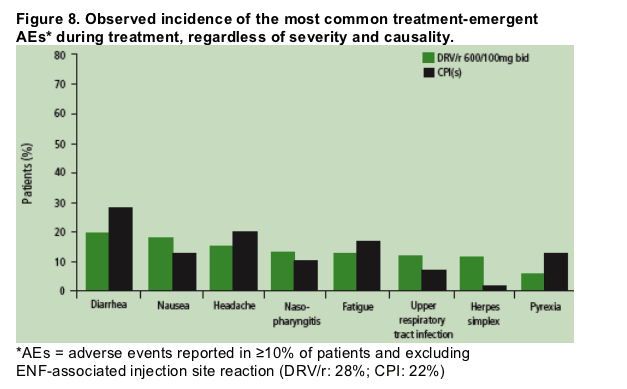
The most common treatment-emergent AEs in both treatment groups, except for ENF-associated injection-site reactions, were diarrhea (20% for DRV/r and 28% for the control PI[s], respectively), nausea (18% and 13%), and headache (15% and 20%) (Figure 8).
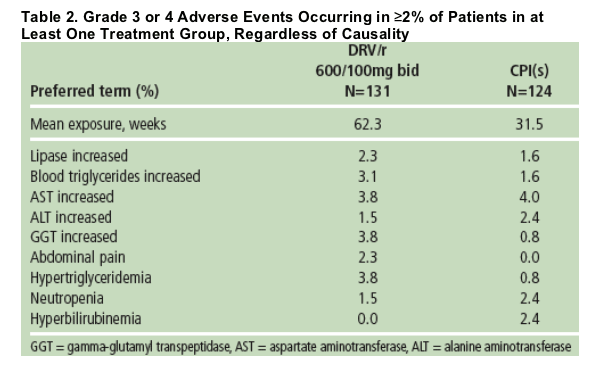
The majority of AEs were mild (grade 1) or moderate in severity; at least one Grade 3 or 4 adverse event was reported in 37% of patients taking DRV/r and 29% taking control PIs (Table 2).
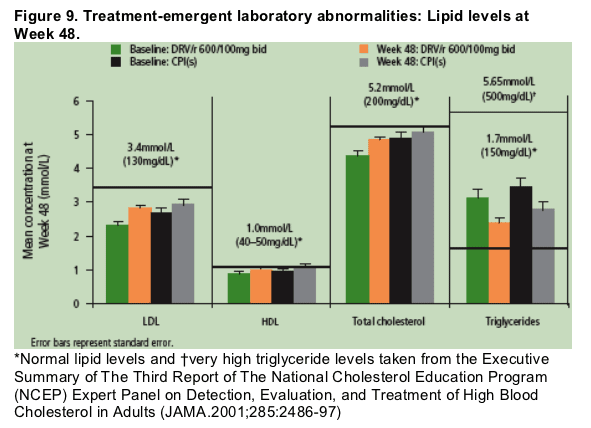
Lipid levels at Week 48 were similar between patients treated with DRV/r and control PIs (Figure 9).
- Mean low-density lipoproteins (LDLs), high-density lipoproteins (HDLs), and total cholesterol levels remained within the normal range as per the National Cholesterol Education Project (NCEP) guideline recommendations.12
- Triglyceride levels were above the normal level (per NCEP guidelines)11 for all patients at baseline; mean triglyceride levels for both DRV/r and control PI patients at Week 48 decreased by 24% and 25%, respectively.
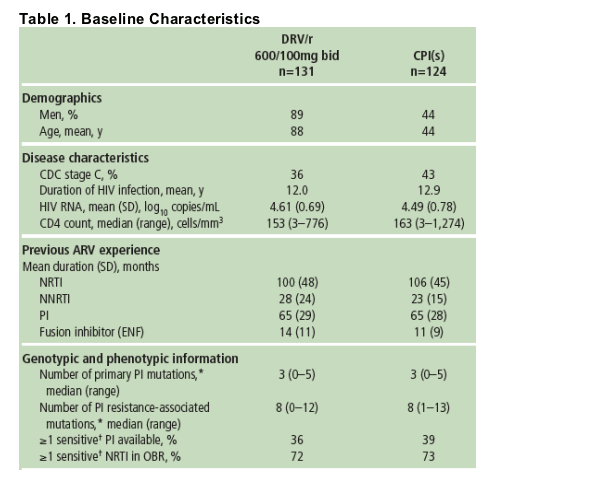
In this analysis, 131/131 (100%) of patients receiving DRV/r and 124/124 (100%) of patients receiving CPI(s) reached Week 48.
The mean duration of prior PI treatment was 65 months, with 3.8 mean prior PIs (4% tipranavir, 81% lopinavir); 19% had prior ENF use. Mean duration of previous ARV exposure was similar between patients receiving DRV and CPIs.
67% of patients had BL FC to DRV ≦10, 21% had FC >10-40, and 12% had FC >40.
According to the IAS-USA 2005 classification,11 patients had a median of three primary PI mutations.
Overall efficacy
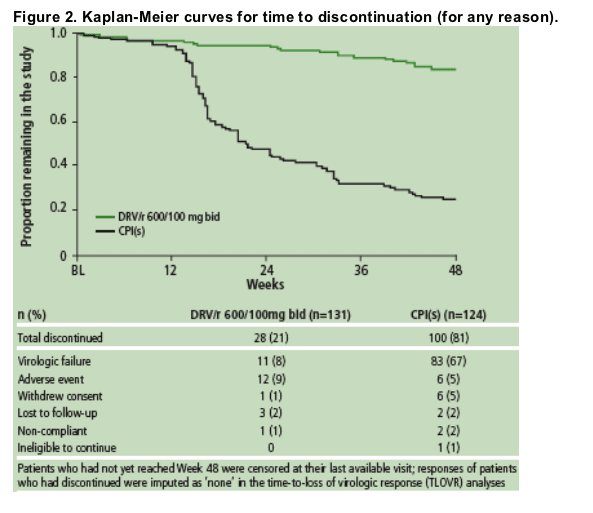
The time to discontinuation in each trial was estimated by means of a Kaplan-Meier analysis (Figure 2). Discontinuations occurred earlier and more frequently in the control group than in the DRV/r groups, with the majority of discontinuations in the control PI group due to virologic failure.
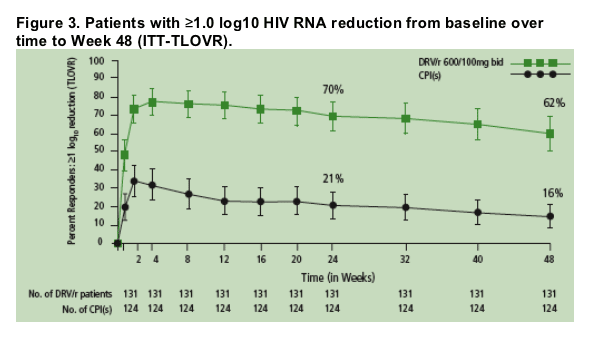
The overall percentage of patients with ≥1 log10 reduction in HIV RNA from baseline was 70% at Week 24 and 62% at Week 48 for DRV/r compared with 21% and 16%, respectively, for the control PI(s) (P<.001 at both Week 24 and Week 48) (Figure 3).
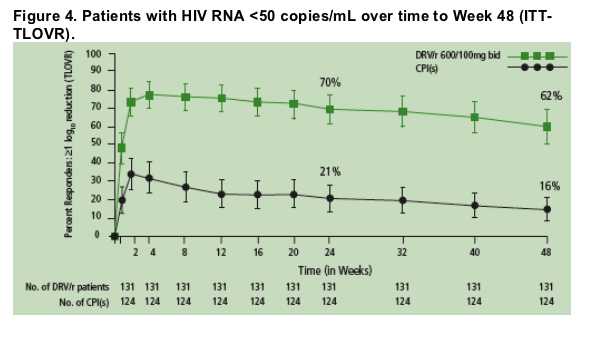
The percentage of patients achieving HIV RNA <50 copies/mL remained similar between Week 24 and Week 48 for DRV/r (45% and 45%, respectively) and the control PI(s) (12% and 11%, respectively) (P<.001 at both Week 24 and Week 48) (Figure 4).
Baseline phenotypic FC in EC50 to DRV predicted response upon multivariate analysis. More patients achieved HIV RNA <50 copies/mL with a baseline DRV FC ≦10 (54%) versus patients with FC >10-40 (34%) and FC >40 (0%) (Figure 5a).
Baseline number of DRV-associated mutations was a strong predictor of response (Figure 5a).
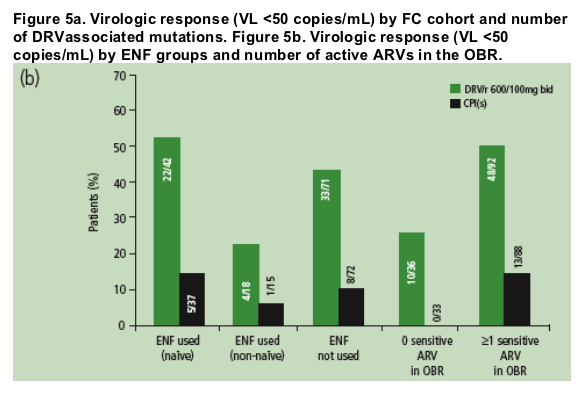
Higher rates of virologic undetectability (HIV RNA <50 copies/mL) were achieved by patients who had ≥1 active ARV in their OBR (Figure 5b).
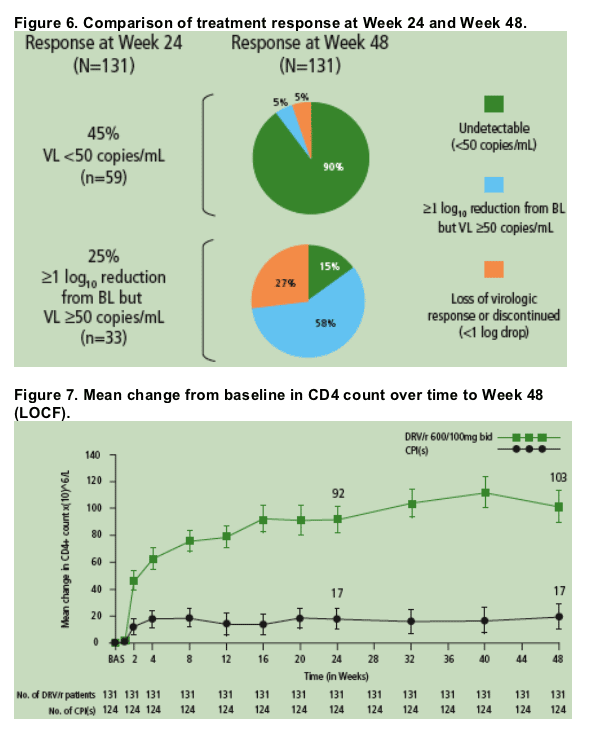
The majority (90%) of patients who achieved HIV RNA <50 copies/mL at Week 24 remained undetectable at Week 48 (Figure 6).
The majority (73%) of patients who achieved ≥1 log10 reduction in HIV RNA at Week 24 but did not achieve full virologic suppression to <50 copies/mL maintained or improved virologic response at Week 48 (Figure 6).
Virologic response was correlated with immunological improvement: the mean change in CD4 cell count from baseline with DRV/r was 92 cells/mm3 at Week 24 and 103 cells/mm3 at Week 48 compared with 17 cells/mm3 at Week 24 and 17 cells/mm3 at Week 48 for the control PI(s) (P<.001 at both Week 24 and Week 48) (Figure 7).
|
| |
|
 |
 |
|
|