 |
 |
 |
| |
Pharmacokinetic interaction between the antiretroviral agents TMC114 and nevirapine, in the presence of low-dose ritonavir
|
| |
| |
Reported by Jules Levin
IDSA, Oct 12-15, 2006, Toronto
Poster 956 Sekar V,1 Lefebvre E,1 Marien K,2 De Pauw M,2 Vangeneugden T,2 Hoetelmans R2
1Tibotec Inc., Yardley, USA; 2Tibotec BVBA, Mechelen, Belgium
NVP is a substrate and inducer of the CYP3A4 enzyme.10 TMC114 and RTV are also substrates and inhibitors of CYP3A4. As a result, concomitant use of NVP with TMC114/r may lower TMC114 plasma concentrations compared to those observed with TMC114/r alone. Furthermore, plasma concentrations of NVP may be affected by TMC114 and RTV co-administration.
The primary objectives of this trial were to assess the effect of TMC114 in combination with low-dose RTV on the pharmacokinetics (PK) of NVP and the effect of NVP on the PK of TMC114 and low-dose RTV, based on historical data. Short-term safety and tolerability of co-administration of TMC114 and low-dose RTV and NVP were also assessed.
Study design
This study (TMC114-C119) was a Phase I, open-label, randomized, crossover trial, designed to consist of 16 HIV-infected volunteers (two panels of eight patients) who were on stable NVP therapy (200mg bid for 316 weeks). A third and fourth panel, each consisting of four other HIV-infected patients on stable NVP therapy, were added to the trial following a protocol amendment due to selection of a tablet formulation for further clinical development of TMC114.
The trial was divided into two sessions of 14 days each (no washout period between sessions). All patients received Treatment A in one session and Treatment B (Panels 1 and 2) or B2 (Panels 3 and 4) in the other session, and continued their usual NVP/NRTI therapy without change. All treatments were administered for 13 days bid, with an additional morning dose on Day 14
- Treatment A: NVP 200mg bid and 32 NRTIs
- Treatment B: TMC114/r 300/100mg bid (oral solution of TMC114) with NVP 200mg bid and >/=2 NRTIs
- Treatment B2: TMC114/r 400/100mg bid (tablet formulation of TMC114) with NVP 200mg bid and >/=2 NRTIs.
TMC114, RTV and NVP had to be taken with food. Where possible, NRTIs were taken at the same time as TMC114; however, dosing schedules of prescribed NRTIs were adhered to.
Safety and tolerability evaluations were recorded throughout the study.
AUTHOR CONCLUSION
The steady-state exposure to NVP (AUC12h) was increased by 27% when NVP was co-administered with TMC114/r, with TMC114 as either a tablet or oral solution, vs NVP alone.
Compared with data on TMC114 and RTV exposure from other studies (TMC114-C207 and C137), the co-administration of NVP and TMC114/r in this study generally resulted in similar exposures to TMC114 and RTV.
The co-administration of TMC114/r and NVP plus NRTIs was generally well tolerated in HIV-1-infected volunteers on a short-term basis.
Based on the small increases in NVP exposure during co-administration with the 300/100mg and 400/100mg bid doses of TMC114/r, a comparable increase is expected when the recommended dose of TMC114/r 600/100 mg bid (with TMC114 in tablet form) is co-administered. The combination of NVP and TMC114/r can be used without dose adjustments.
PK analysis and study design
After the morning dose on Day 14 of Treatment A, a 12-hour PK profile of NVP was determined. After the morning dose on Day 14 of Treatment B or B2, PK profiles of TMC114, RTV and NVP were assessed up to 12 hours post-dose.
The PK results of NVP on Day 14 of Treatment B and Treatment B2 were compared with the PK results of NVP on Day 14 of Treatment A.
Severe, life-threatening, and in some cases, fatal hepatotoxicity has been reported in healthy volunteers as well as in HIV-1-infected patients treated with NVP.10 The current trial was performed in HIV-1-infected patients, who were on a stable NVP treatment.
It was considered unethical in this setting to administer TMC114/r in the same HIV-1-infected volunteers for 14 days without co-administration of NVP (as NVP was a component of their ongoing treatment for HIV infection). Therefore, the pharmacokinetics of TMC114 and RTV on Day 14 of Treatment B were compared to corresponding PK data from the TMC114-C207 trial in HIV-1-infected volunteers (oral TMC114 solution and same dosing regimen of TMC114/r administered to 13 HIV-1-infected volunteers for 13 days with an additional morning dose on Day 14).
The pharmacokinetics of TMC114 and RTV on Day 14 of Treatment B2 were compared to corresponding PK data from the TMC114-C137 trial in healthy volunteers (tablet formulation at the same dose of TMC114/r administered to eight healthy volunteers for 6 days with an additional morning dose on Day 7).
Plasma concentrations of TMC114, RTV and NVP were determined by validated liquid chromatography mass spectrometry/mass spectrometry methods.
To ensure steady-state was reached on Day 14, when full PK profiles were determined, predose plasma concentrations (C0h) were determined during treatment.
The least square means (LSM) of the primary PK parameters for each treatment group were estimated with a linear mixed effects model, including treatment, period and sequence as fixed effects and individual as a random effect. The 90% confidence intervals (CIs) around the LSM ratios were also calculated.
RESULTS
Patient disposition
Sixteen patients were planned for inclusion in Panels 1 and 2; however, due to under-recruitment only 11 patients (seven and four patients, respectively) were included. All eight planned patients were included in Panel 3 and 4 (four in each).
Of the 19 randomized patients, 15 completed the trial. Four patients discontinued: two due to withdrawal of consent, one due to an adverse event (AE), classified as serious, and one due to non-compliance. The median age of these patients was 43 years (range 33-56 years) and 14 (74%) were male.
PK results for NVP
The mean plasma concentration profiles of NVP revealed that administration of TMC114/r (tablet or oral solution) and NVP resulted in higher NVP systemic exposure (area under the curve [AUC]) vs NVP alone (Figure 1).
Predose plasma concentrations of NVP were comparable between Days 7, 12, 13 and 14, showing achievement of steady-state prior to PK assessments.
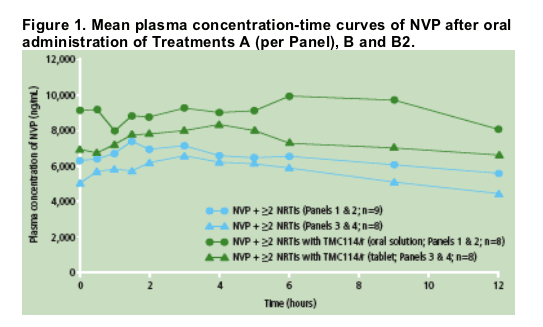
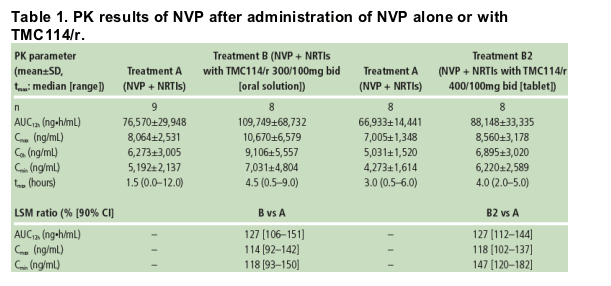
Based on the LSM ratio, the mean NVP exposure (AUC from time of intake to 12 hours post-dose, AUC12h) was increased by 27% when TMC114/r (tablet or oral solution) and NVP were administered vs NVP alone (Table 1).
The mean minimum plasma concentration (Cmin) of NVP was increased by 47% when co-administered with TMC114/r (400/100mg bid; TMC114 tablet) vs NVP alone. When TMC114/r (300/100mg bid; TMC114 oral solution) and NVP were co-administered, NVP Cmin increased by 18%. The maximum plasma concentration (Cmax) of NVP was only slightly increased by either formulation/dose of TMC114.
PK results for TMC114 and RTV
Mean plasma concentration profiles of TMC114 and RTV after administration of TMC114/r (tablet or oral solution) and NVP were determined (Figure 2).
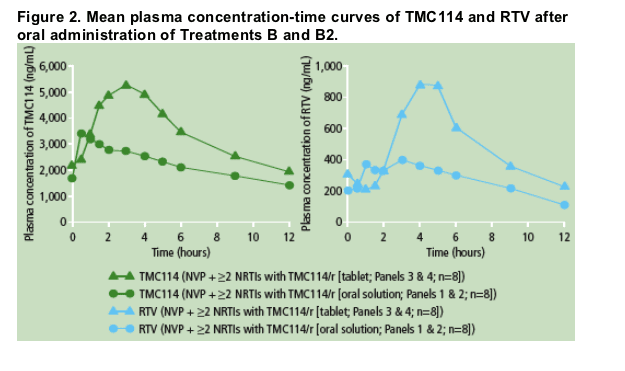
Predose plasma concentrations of TMC114 were comparable between Days 7, 12, 13 and 14; this shows that steady-state conditions were achieved prior to PK assessments. PK results for TMC114 are shown in Table 2.
The statistical analysis performed for TMC114 pharmacokinetics was a between-study comparison; the results are therefore an indication of the potential effect of NVP on TMC114/r pharmacokinetics only.
Based on the LSM ratio, the mean TMC114 Cmin and AUC12h increased by 23% and 9%, respectively, and mean Cmax decreased by 16% when TMC114/r (300/100mg bid; TMC114 oral solution) and NVP were co-administered vs TMC114/r alone (TMC114-C207; Table 2). These changes did not reach
statistical significance.
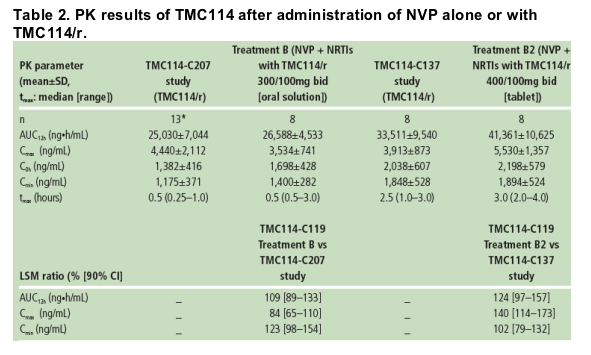
The mean TMC114 Cmax increased by 40% when TMC114/r (400/100mg bid; TMC114 tablet) and NVP were co-administered vs TMC114/r alone (TMC114-C137; Table 2). However, TMC114 AUC12h and Cmin were only slightly increased in the presence of NVP.
For RTV, based on the LSM ratio, the mean AUC12h, Cmax and Cmin decreased by 16%, 14% and 17%, respectively, when TMC114/r (300/100mg bid; TMC114 oral solution) and NVP were co-administered vs TMC114/r alone (TMC114-C207).
When TMC114/r (400/100mg bid; TMC114 tablet) and NVP were co administered, mean RTV AUC12h and Cmax increased by 10% and 23%, respectively, and Cmin decreased by 2% vs TMC114/r alone (TMC114-C137). Different trials were used to estimate the effect of NVP on RTV pharmacokinetics.
Safety
Of the 19 patients randomized, 15 completed the trial. One patient discontinued the trial after subarachnoid hemorrhage, which was reported as a serious AE (SAE); this was considered to be unrelated to treatment by the investigator and occurred during the follow-up period 14 days after the last trial medication intake. This patient was diagnosed with left and right middle cerebral artery aneurysms. No other SAEs or AEs leading to discontinuation were reported during this study.
Overall, 14 patients (74%) reported at least one AE during the trial; most were grade 1 or 2.
Only one AE - one case of diarrhea during Treatment B - was reported as very likely related to the trial medication.
AEs reported by more than one patient during the trial are shown in Table 3. Most commonly reported AEs were diarrhea (seven patients; 37%) and headache (five patients; 26%).
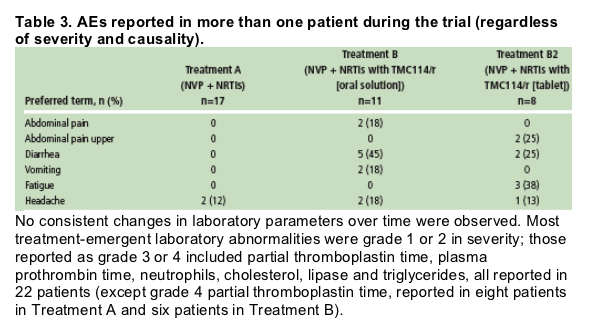
No consistent changes in laboratory parameters over time were observed. Most treatment-emergent laboratory abnormalities were grade 1 or 2 in severity; those reported as grade 3 or 4 included partial thromboplastin time, plasma prothrombin time, neutrophils, cholesterol, lipase and triglycerides, all reported in 22 patients (except grade 4 partial thromboplastin time, reported in eight patients in Treatment A and six patients in Treatment B).
AUTHOR CONCLUSIONS
The steady-state exposure to NVP (AUC12h) was increased by 27% when NVP was co-administered with TMC114/r, with TMC114 as either a tablet or oral solution, vs NVP alone.
Compared with data on TMC114 and RTV exposure from other studies (TMC114-C207 and C137), the co-administration of NVP and TMC114/r in this study generally resulted in similar exposures to TMC114 and RTV.
The co-administration of TMC114/r and NVP plus NRTIs was generally well tolerated in HIV-1-infected volunteers on a short-term basis.
Based on the small increases in NVP exposure during co-administration with the 300/100mg and 400/100mg bid doses of TMC114/r, a comparable increase is expected when the recommended dose of TMC114/r 600/100 mg bid (with TMC114 in tablet form) is co-administered. The combination of NVP and TMC114/r can be used without dose adjustments.
|
| |
|
 |
 |
|
|