 |
 |
 |
| |
Optiming Response Rates to Peg/RBV in Coinfected Patients: increased Ribavirin dosing; extending therapy duration
|
| |
| |
Reported by Jules Levin
Jan 13, 2005
So, what was interesting today at the 2nd Intl HIV and Hepatitis Coinfection Workshop in Amsterdam Jan 12-14, 2006?
The Coinfection Workshop is the only conference dedicated to coinfection treatment. It is a good venue for open discussion of treatment principles both established and experimental. Of most interest today was the latest update from Vincent Soriano (Spain) on his study using 1000/1200 mg of ribavirin in coinfected patients, the PRESCO Study. The main coinfection studies that had been conducted, APRICOT & RIBAVIC used 800 mg daily of ribavirin because of the concern for potential side effects and toxicities, but in HCV monoinfection ribavirin is dosed with 800, 1000, or 1200 mg per day based on a patient's weight. Of note, interim results from PRESCO study found more patients with non-genotype 1, as well as genotype 1 patients, achieved SVRs when using higher ribavirin doses (1000/1200 mg/day). These study results suggest ribavirin dosing of 1000/1200mg achieve better SVR rates than 800 mg/day in coinfected patients. The Fried Pegasys Study found 800 mg of ribavirin in monoinfected patients achieved equal rates of SVR in genotype 2/3 as when using higher ribavirin doses. Soriano's talk is divided into 2 topics: 1- Enhancement of Early Viral Response, and 2-Reduction of Relapse Rates. The title of Soriano's talk was:
"How to optimize response rates in HCV/HIV-coinfected patients"
Vincent Soriano
Department of Infectious Diseases
Hospital Carlos III, Madrid
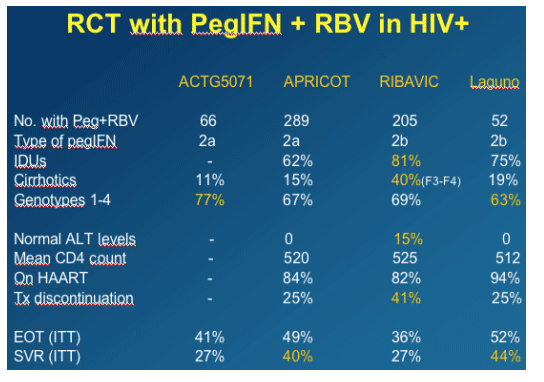
Randomized Clinical Trials in Coinfection
Main predictors of SVR
--HCV genotype
--Baseline HCV-RNA
--Adherence (80/80/80)
--W4 virological response (best PPV of SVR)
What we can do to improve SVR in HIV-HCV co-infection?
Select candidates correctly, improve adherence, and manage appropriately side effects
Enhance early virological response
--Induction doses: pegIFN and/or RBV
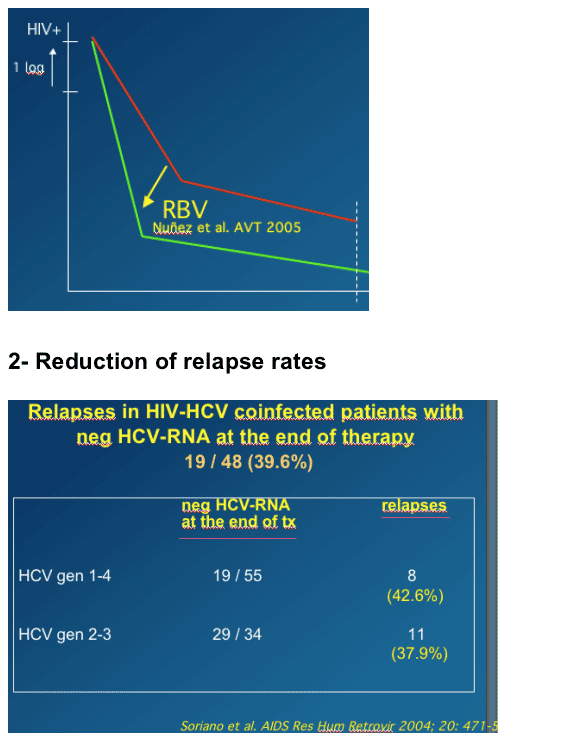
Reduce relapse rates
--Extending the length of therapy
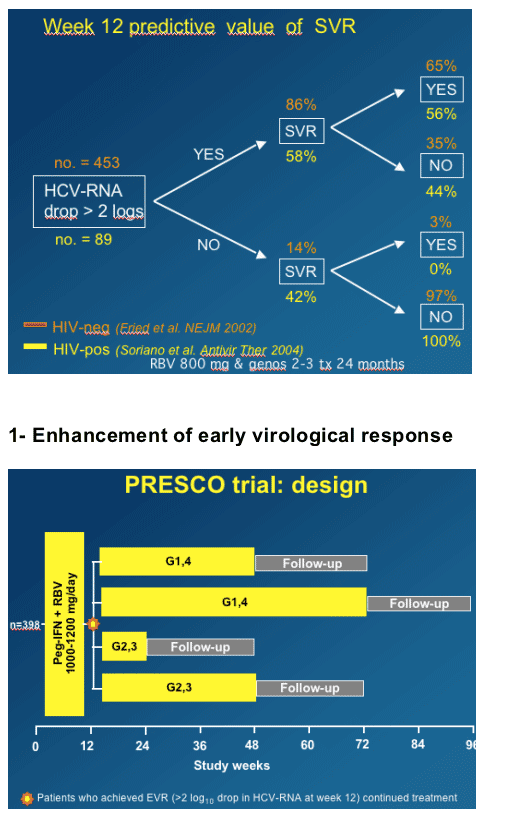
As you can see from this graph, 65% of monoinfected patients with 12-Week early Viral Response achieved an SVR, while in coinfected patients 56% of patients with 12-week Early Viral Response achieved an SVR.
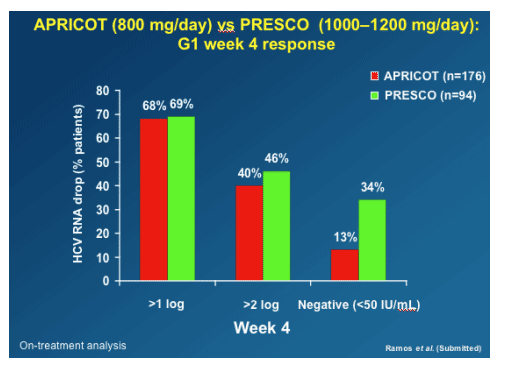
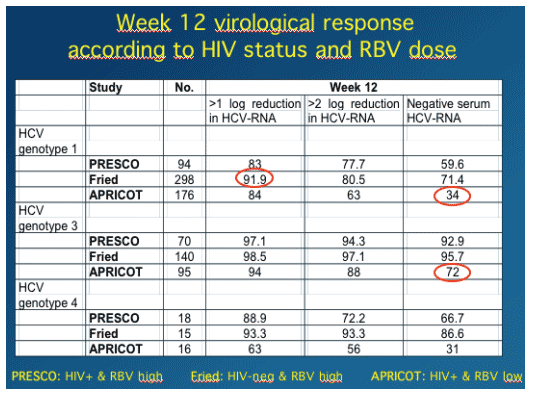
You can see in this table that at week 4 genotype 1 patients in PRESCO (receiving 1000/1200 mg/day RBV) had a higher rate achieving <50 IU/mL than patients in APRICOT who received 800mg daily RBV. As well, 46% vs 40% achieved >2 log viral load reduction in PRESCO vs APRICOT.
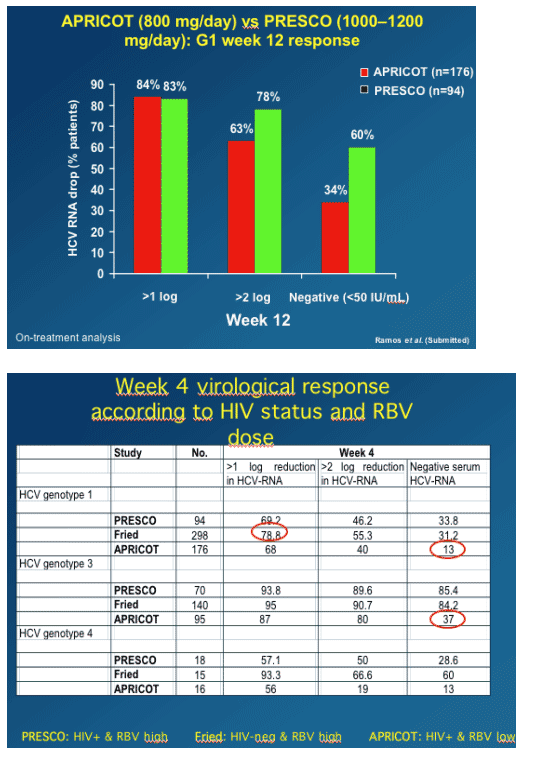
WEEK 12 RESULTS
A greater percentage of genotype 1 patients in PRESCO vs APRICOT achieved >2 log viral load reduction at week 12 (78% vs 63%) and also achieved <50 IU/ml (60% vs 34%).
Clinical interpretation
--HIV impacts negatively on the early virological response to HCV therapy.
--Prescription of appropriate (high) RBV doses increase early virological response mainly in genotypes 1-4 but also in genotypes 2-3.
Impact of RBV exposure
Ribavirin doping (exposure) alter initiating therapy correlats with viral load reductions.
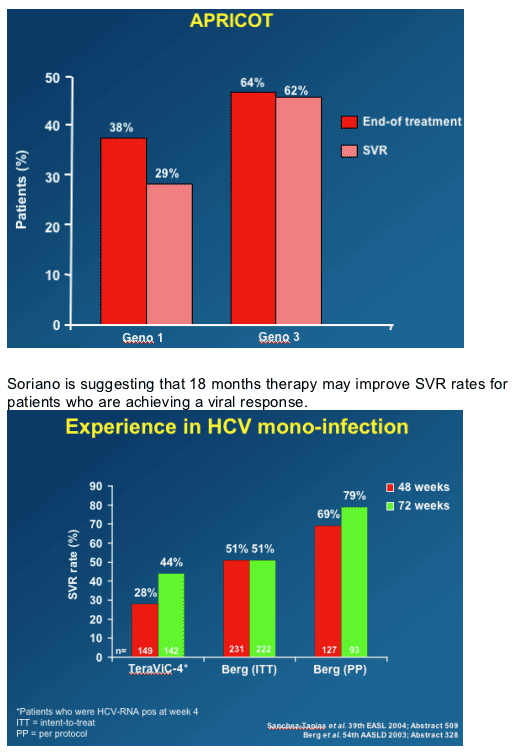
You can see the relapse in genotype 1 in APRICOT: 38% end of treatment response and 29% sustained viral response rate.
Clinical interpretation
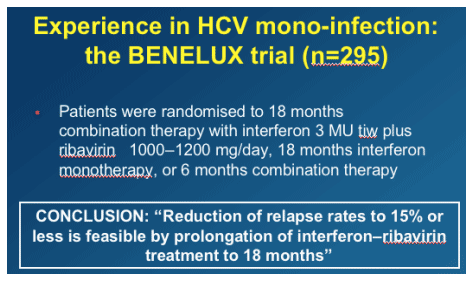
Extending treatment mainly for genotypes 2-3 (from 6 to 12 months) and perhaps in genotypes 1-4 (from 12 to 18 months), mainly in those with high baseline HCV-RNA, might allow to reach response rates in HCV-HIV co-infected patients approaching those seen in HCV-monoinfected individuals. (Note from Jules Levin: of course, this applies to patients who achieve a good viral response rate within the first 12-24 weeks.
|
|
| |
| |
|
 |
 |
|
|