 |
 |
 |
| |
Selection of NNRTI resistant HIV-1 after discontinuation of a virologically suppressive regimen: standard genotype test does not detect all resistance
|
| |
| |
Reported by Jules Levin
XV HIV Drug Resistance Workshop
Sitges, Spain
June 13-17, 2006
CB Hare 1, J Mellors 2, A Krambrink3, Z Su3, D Skiest4, D Margolis 5, S Patel 3, D Barnas3, L Frenkel6, R Coombs6, F Aweeka1, G Morse7, DW Haas8, R Kim8, V Boltz 9, S Palmer9, J Coffin9 and DV Havlir 1
1University of California, San Francisco, CA
2University of Pittsburgh, PA
3SDAC/Harvard School of Public Health, Boston, MA
4Baystate Medical Center, Springfield, MA
5University of North Carolina, Chapel Hill, NC
6University of Washington, Seattle, WA
7State University of Buffalo, NY
8Vanderbilt University, Nashville, TN
9HIV Drug Resistance Program, National Cancer Institute at
Frederick, MD, USA
Note from Jules Levin: There are 2 important points from this study. First, this was the second study presented at the Workshop reporting that standard genotype tests failed to identify drug resistance that more sensitive genotypic tests picked up. Second, in this ACTG substudy, patients who had undetectable HIV viral load (<400 copies/ml) discontinued HAART. They stopped the NNRTI up to two days before the remainder of the regimen. NNRTI drug resistance was found in 20% of the patients after stopping HAART. But NNRTI drug resistance was not found in some patients without using the sensitive genotype test; 6 patients had NNRTI drug resistance that was not detected by standard genotypic testing, but was found with sensitive testing. Five patients who restarted the same HAART regimen experienced viral failure: 2 failures had resistance by standard genotype test and 3 failures had no resistance demonstrated prior to restarting ART. So, firstly, stopping therapy when on NNRTI regimen risks the development of drug resistance for several reasons but particularly because NNRTIs stay in the blood at decreasing levels over the course of days. It is impossible to predict how long they stay in the blood for an individual. Adding a protease regimen for two weeks before interrupting therapy has been recommended as a way to prevent resistance, but this method is not foolproof & is not easy to implement properly. Second, when stopping therapy a patient may have drug resistance not detected by standard genotype testing and if restarted on the same regimen if resistance is present this increases the risk for treatment failure. A third point from this study is that patients who had viral load between 50 and 400 were at greater risk for developing resistance & therefore subsequent viral failure, particularly if restarting the same regimen.
Author Conclusion:
Individuals who discontinue NNRTI-containing ART while virologically suppressed are at substantial risk (20%) of demonstrating NNRTI resistance
at virological rebound that may persist in plasma for months. The highest risk of resistance is associated with low-level viral replication (HIV RNA 51-400
c/ml) at treatment discontinuation (45% resistance). The effect of this resistance on response to subsequent NNRTI-containing ART warrants further study.
BACKGROUND: The frequency of emergence of drug-resistant virus after discontinuation of suppressive antiretroviral therapy (ART) is undefined.
METHODS: Study subjects were on NNRTI-containing regimens with HIV RNA <400 c/ml enrolled in ACTG A5170, a prospective 2-year treatment discontinuation study of individuals with CD4>350 cells/mm3. Other components of ART were stopped within 2 days of NNRTI. At first RNA >5000 c/ml after ART discontinuation, resistance in plasma was measured by standard genotype (SG) and allele-specific PCR (ASP) (sensitive resistance test). Predictors of NNRTI resistance at rebound examined include prior regimens, HIV RNA, CD4, baseline PBMC resistance by oligonucleotide ligation assay, NNRTI drug concentrations and CYP2B6 polymorphisms.
RESULTS:
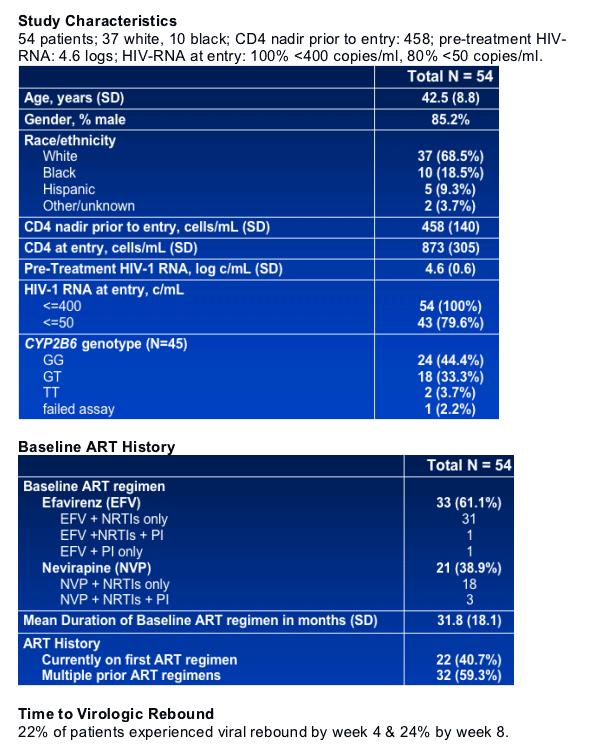
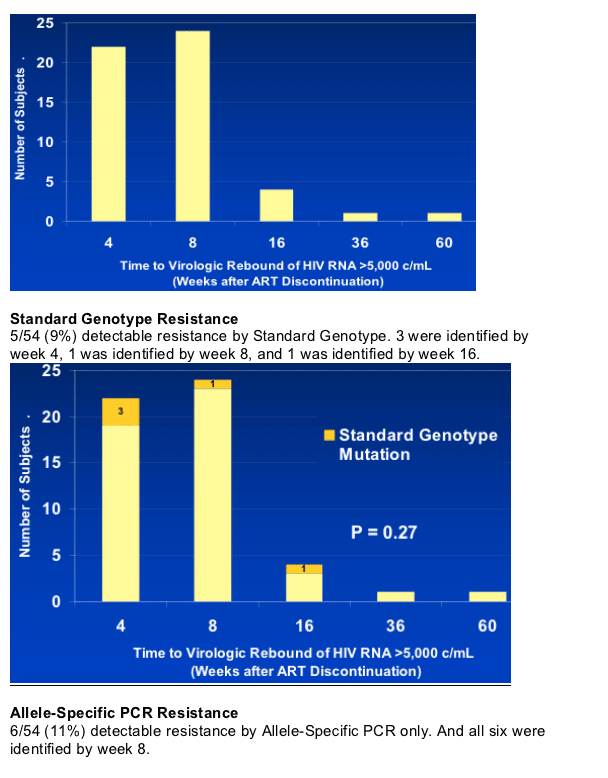
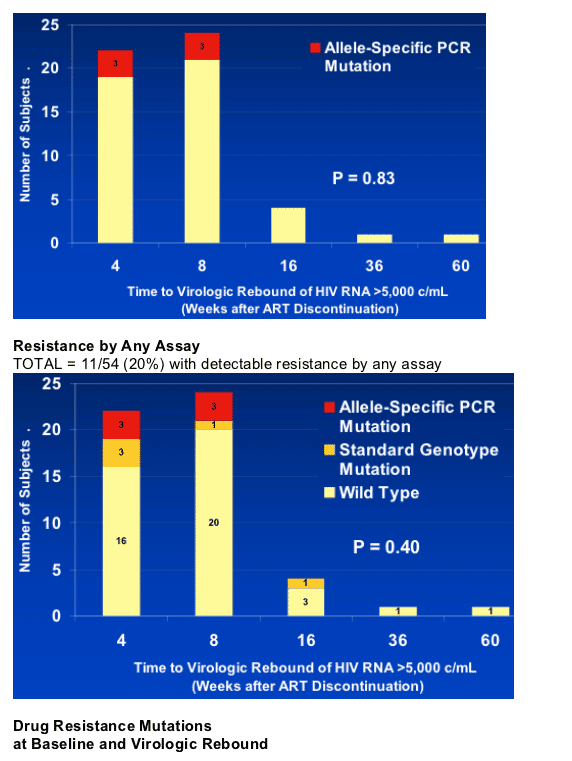
NNRTI resistance mutations were detected at virological rebound in 11 of 54 (20%) subjects (33 on efavirenz, 21 on nevirapine): 5 (9%) by SG; and 6 (11%) by ASP.
Resistance mutations by SG were all K103N; by ASP - K103N (4), Y181C (1), and K103N/Y181C (1).
After ART discontinuation, RNA >5000 c/ml was reached at week-4 in 23 subjects, Week-8 in 25, and ≥week-16 in 6.
Subjects with baseline RNA 51-400 c/ml had a 3.3 fold (95% CI=1.3, 8.5) increased risk for resistance at rebound compared to subjects with RNA ≦50 c/ml. NNRTI resistance emerged in 45% of subjects with baseline RNA 51-400 c/ml versus 14% with RNA ≦50 c/ml (P=0.03).
PBMC resistance was more frequent among subjects with RNA 51-400 c/ml at baseline and was associated with a 2.9 fold (95% CI=1.1, 7.4) increased risk for
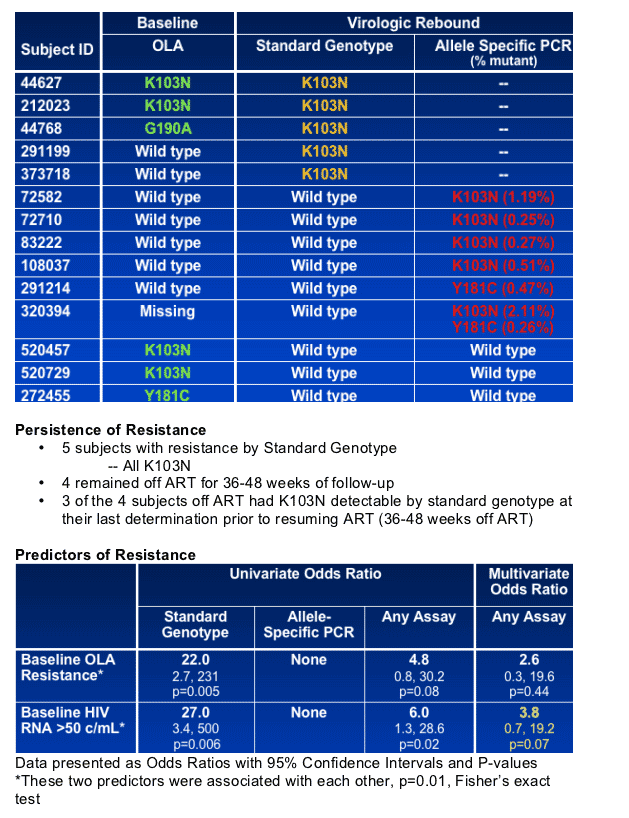
resistance at rebound. Resistance persisted for the duration of treatment interruption (36-48 weeks) in 3 of 5 subjects with mutations by SG. Five virological failures occurred among 23 subjects who restarted ART during observation; all 5 restarted the same prior regimen and 2 of 5 had NNRTI resistance.
Selection of Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) Resistant HIV-1 After Discontinuation of a Virologically Suppressive Regimen: ACTG NWCS 239
Reported by Jules Levin
XV HIV Drug Resistance Workshop
Sitges, Spain
June 13-17, 2006
Presented by Brad Hare, MD, University of California, San Francisco
AUTHOR SUMMARY
Individuals who discontinue NNRTI-containing ART while virologically suppressed are at substantial risk (20%) of demonstrating NNRTI resistance at virologic rebound.
This resistance may persist for months in plasma by standard genotyping.
Resistance assays with increased sensitivity over standard genotyping may detect increased frequency of resistance.
The highest risk of resistance (45%) is associated with low-level viral replication (HIV RNA 51-400 c/ml) at treatment discontinuation.
Implications
Discontinuation of NNRTI regimens during periods of low-level viremia should be avoided.
Staggering NNRTI and non-NNRTI discontinuation by 2 days does not completely protect against resistance.
Genotype testing up to one year after NNRTI discontinuation may be of clinical utility.
The effect of this resistance on response to subsequent NNRTI-containing ART warrants further study.
Background
- Patients may discontinue virologically suppressive antiretroviral therapy (ART) regimens for reasons including patient choice or toxicity1
- Upon ART discontinuation, drug-resistant virus may emerge as the predominant strain or as minor variants2
- The frequency of emergence and clinical significance of drug-resistant virus in this setting is undefined
Study Objectives
Primary Objective:
To estimate the frequency of NNRTI resistance mutations among persons discontinuing virologically suppressive NNRTI-containing ART regimens
Secondary Objectives:
-- To determine the timing of emergence and persistence of NNRTI resistance mutations
-- To identify predictors of resistance in this setting
-- To assess the impact of detectable resistance on response to subsequent NNRTI treatment
Study Design
Virologic Sub-study of ACTG A5170: Single-arm observational study of ART discontinuation with clinical and laboratory endpoints1
Inclusion criteria:
-- Nadir CD4 > 250; CD4 at enrollment > 350
-- On continuous ART for >6 months
-- Taking NNRTI at time of ART discontinuation
-- HIV RNA < 400 c/mL at entry
Subjects instructed to discontinue NNRTI 2 days prior to other drugs.
Methods
Baseline resistance:
-- HIV DNA by Oligonucleotide Ligation Assay (OLA) from PBMC1
Resistance at viral rebound: (HIV RNA >5,000 c/mL)
-- Standard population-based genotype
If present, re-tested throughout period of study observation
-- Allele specific RT PCR for codons 103 and 1812
Tested only if standard genotype showed no resistance
Pharmacokinetics:
NVP or EFV drug concentrations at baseline and Week-4 after treatment discontinuation, followed until undetectable
Pharmacogenomics:
CYP2B6 polymorphisms
Virologic Response
After ART Re-Initiation
23 Subjects restarted ART during study observation
- Restart with NNRTI, N = 18
- Restart with exact same regimen, N = 13
5 Virologic failures observed
- All failures among the N = 13 who started the exact same regimen
- 2 failures had resistance by Standard Genotype prior to restarting ART (both K103N)
- 3 failures had no resistance demonstrated prior to restarting ART
|
| |
|
 |
 |
|
|