 |
 |
 |
| |
De-selection of the I50V mutation occurs in clinical isolates during Aptivus/r (tipranavir/ritonavir)-based therapy
|
| |
| |
Reported by Jules Levin
XV HIV Drug Resistance Workshop
June 13-17, 2006
Sitges, Spain
R Elston1, J Scherer2, D Hall2, J Schapiro3, R Bethell1, V Kohlbrenner2, D Mayers2
1Boehringer Ingelheim (Canada) Ltd Research and Development, Laval, Quebec, Canada. 2Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, Connecticut, USA. 3Stanford University, Stanford, California, USA.
Poster Number 92
Author Conclusions
- The I50V mutation has been reported to be selected by amprenavir and lopinavir.
- The I50V mutation is associated with increased susceptibility to TPV.
- PI-experienced patients harboring virus with an I50V mutation at baseline were
more likely to have a clinical response compared to patients receiving comparator PIs.
- If the I50V mutation is present at baseline during Aptivus/r-based therapy, it is
de-selected upon virological rebound.
Introduction
The I50V protease mutation is a major mutation (Johnston 2005) that was first
described following in vitro selection experiments with the HIV-1 protease
inhibitor (PI) amprenavir (APV, Partaledis 1995). Subsequently the I50V mutation
has been observed to develop in the clinical setting following virological failure of
the PIs amprenavir (Maguire 2002), lopinavir (LPV, Mo 2005) and more recently
fosamprenavir (FPV, Sax 2005). The importance of the I50V mutation for PIs with
chemical structures closely related to amprenavir has also been highlighted by
several studies, for example, the presence of the I50V mutation at baseline has
been associated with reduced virological response to darunavir (TMC-114,
Bethune 2006). In addition the I50V mutation was selected during in vitro
passage experiments with GW640385 (brecanavir, Yates 2006) and TMC-126
(Yoshimura 2002).
Tipranavir (Aptivus), in combination with low-dose ritonavir, is the latest PI to be
approved for clinical use in highly treatment-experienced patients.
Tipranavir (TPV) has a distinct resistance profile selecting both known and unique resistance protease mutations (e.g. V82L). Cross resistance studies have
confirmed the ability of TPV to inhibit multi-PI resistant clinical isolates
(Larder 2000).
The characterization of mutations responsible for increased or decreased
susceptibility to TPV is critical for further optimization of Aptivus/r use. Three
recent studies have identified protease mutations associated with increased or
decreased susceptibility to TPV (Parkin 2006, Batchelor 2006, Hall 2006). The
D30N, I50V and I54L mutations were identified in all three studies as being
associated with increased susceptibility to TPV. The objective of this analysis
was to determine the evolution of the I50V mutation in patients treated with an
Aptivus/r-containing regimen.
Methods and materials
Clinical isolates were obtained from patients participating in the RESIST 1
(N=620) and RESIST 2 (N=863) clinical trials. The RESIST 1 and RESIST 2
studies were open label, randomized clinical trials designed to evaluate the
efficacy of Aptivus/r (500mg/200mg twice daily) to a comparator ritonavir
boosted PI regimen (CPI/r). Patients were randomized 1:1 to receive Aptivus/r
or CPI/r. Patients had to be NRTI and NNRTI experienced and had to have
received at least two prior PIs.
All patients had a baseline genotype. Genotypes were obtained by commercially
available population sequencing. For the purposes of this analysis, genotypes
containing mixtures at position I50 at baseline were excluded. Major protease
inhibitor mutations were defined as D30N, V32I, L33F, M46I/L, G48V, I50V/L,
V82A/F/L/T/S, I84V, N88S and L90M (Johnston 2005). NRTI mutations were
defined as M41L, E44D, A62V, K65R, D67N, K70R, L74V, F77L Y115F, V118I,
Q151M, M184V, L210W, T215Y/F, K219Q/E (Johnston 2005). NNRTI mutations
were defined as L100I, K103N, V106A/M, V108I, Y181C/I, Y188L, G190S/A,
P225H, P236L (Johnston 2005).
Baseline phenotype (Virco AntivirogramTM assay) was ordered for a stratified
random sample (4:1 Aptivus/r:CPI/r) of 500 patients from RESIST-1 and
RESIST-2. Patients randomized to receive Aptivus/r and experiencing virological failure also had phenotypes ordered at virological failure.
Results
1.0 Prevalence of I50V/L mutations at baseline
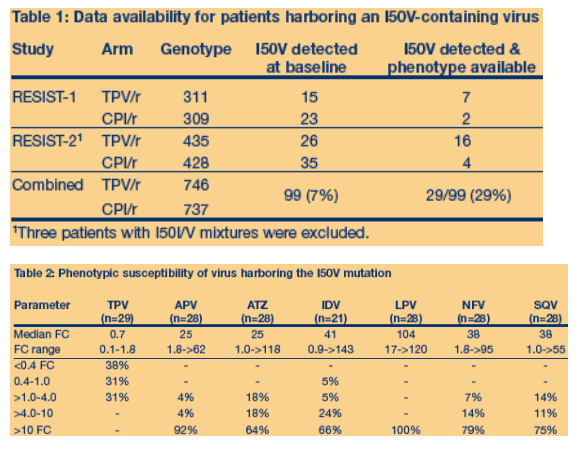
- Overall 7% (99/1483) of patients harbored virus with an I50V mutation at entry
into RESIST 1 and 2. Baseline phenoype data was available for 29% of these
isolates (Table 1).
- Three additional patients harbored virus with the atazanavir (ATZ) associated
I50L mutation. Phenotype was available for virus from one of these patients
and identified high level resistance to ATZ (>102-Fold Change (FC)) and
nelfinavir (NFV, 33.5-FC), but increased susceptibility to TPV (0.2-FC), APV
(0.4-FC) and indinavir (IDV, 0.3-FC).
1.1 Virological response of patients infected with an I50V containing virus
- A greater median reduction in baseline viral load was observed at week 24
in patients receiving Aptivus/r (-1.44 log10 copies/mL, n=41) than CPI/r
(-0.20 log10 copies/mL, n=58) [P <0.0001, based on a Wilcoxon rank sum test].
- A greater median reduction in baseline viral load was observed at week 24
in patients receiving Aptivus/r in the presence of a baseline I50V mutation
(-1.44 log10 copies/mL, n=41) compared to in the absence of an I50V mutation
(-0.72 log10 copies/mL, n=58) [P = 0.0345, based on a Wilcoxon rank
sum test].
1.2 Characterization of virus harboring the I50V mutation for which
genotypic and phenotypic data was available (n=29)
- Isolates harboring the I50V mutation harbored multiple major PI mutations
(median n=4): D30N (0%), V32I (0%), L33F (41%), M46I/L (62%), G48V (45%),
I50V (100%), V82A/F/L/T/S (83%; A (n=21), T (n=2), S (n=1), F/L (n=0)), I84V
(0%), N88S (0%) and L90M (28%).
- Isolates harboring the I50V mutation had extensive NRTI mutations (median
n=6) and NNRTI mutations (median n=1) including: M41L (83%), D67N (72%),
K70R (34%), K103N (45%), M184V (59%), L210W (52%), T215F/Y (97%),
K219K/E (45%).
- The majority (69%) of isolates harboring the I50V mutation had evidence of increased susceptibility to TPV (<1.0 FC) (Table 2).
- In contrast, the majority of isolates had >10-fold reductions in susceptibility
to the other PIs. Only 2/28 isolates had <40-fold reduction in susceptibility to
LPV. Only one isolate had increased susceptibility to another PI (IDV).
1.3 Effect of the baseline I50V mutation on TPV susceptibility in the
presence of other TPV-associated resistance mutations
- The most frequently observed protease mutations which developed after
virological failure of an Aptivus/r-based regimen in the RESIST studies are
L33F/I/V, V82T and I84V (FDA package insert).
- The presence of the I50V mutation with the L33F mutation at baseline
increased susceptibility to TPV: L33F+I50V [median FC=0.3 (0.1 to 1.8-FC,
n=12)]; L33F-I50V [median FC=1.8 (0.3 to 115-FC, n=60)].
- The presence of the I50V mutation with the V82T mutation at baseline
increased susceptibility to TPV: V82T+I50V [median FC=0.75 (0.5 to 0.8-FC,
n=2)]; V82T-I50V [median FC=3.0 (0.5 to 7.6-FC, n=20)].
- No isolate harbored both I50V and I84V mutations.
1.4 Evolution of baseline I50V mutation following virological rebound:
- Twenty three patients harbored virus with an I50V mutation at baseline, had
phenotype available and received an Aptivus/r based regimen.
- Of the nine patients that experienced virological rebound and had genotypic
data available, all nine de-selected the I50V mutation (Figure 1).
- The majority of isolates also developed the V82T mutation (7/9) in combination
with the I84V mutation (6/7).
- Selection of the V82TħI84V mutations together with de-selection of the I50V
mutation resulted in decreased susceptibility to TPV (baseline median FC of
0.7 increased to 18-FC (n=5)), but increased the susceptibility to APV (baseline
median FC of 21 decreased to 5-FC, (n=5)).
1.5 Evolution of baseline D30N and I54L mutations following virological
rebound
- The D30N and I54L mutations have also been associated with increased
susceptibility to TPV.
- The D30N and I54L mutations were present at lower frequency than the I50V
mutation at baseline: D30N (3%; 47/1483) and I54L (4%; 65/1483).
- Phenotypic data was available for eleven baseline isolates with the D30N
mutation. The median FC in susceptibility to TPV was 0.4 (range 0.2-2.1).
- Phenotypic data was available for fourteen baseline isolates with the I54L
mutation. The median FC in susceptibility to TPV was 1.2 (range 0.2-7.7).
- No patient with the D30N at baseline failed Aptivus/r therapy and
consequently the evolution of the D30N mutation cannot be monitored.
- Two patients harbored virus with the I54L mutation at baseline, received
APTIVUS/r therapy and experienced virological rebound. In both cases upon
virological failure the I54L mutation was replaced with the I54V mutation and
TPV resistance was detected (FC of 2.5 increased to 41; FC of 1.1 increased
to 18.7).
Discussion
The I50V mutation is a mutation that is associated with resistance and/or decreased virological response to not only currently approved PIs (APV, FPV and LPV) but also to PIs currently in clinical development (darunavir (TMC-114) and brecanavir (GW 640385)).
In contrast to other protease inhibitors, the presence of the I50V mutation was
associated with increased susceptibility of isolates to TPV. In this analysis,
approximately two thirds of the isolates with the I50V mutation had increased
susceptibility to TPV. None of the twenty-nine I50V containing isolates had greater than a 2-fold reduction in susceptibility to TPV despite the presence of multiple PI mutations. This was in marked contrast to other licensed PIs where between 64%-100% of these isolates had >10-fold reduction in susceptibility.
In agreement with the phenotypic susceptibility, in the presence of a baseline
I50V mutation a better virological response at week 24 was observed for patients
that received Aptivus/r than CPI/r. Interestingly, patients receiving Aptivus/r
and harboring virus with an I50V mutation had a greater median viral load
reduction compared to those without the I50V mutation. Further investigation is
however required to establish whether patients infected with virus having increased susceptibility to TPV have a superior response to Aptivus/r therapy.
Some patients with an I50V mutation at baseline did however fail on an Aptivus/r
regimen and further investigation into other factors influencing the virological response of these patients is warranted e.g. the activity of the background regimens, baseline viral load etc. Consistent with the I50V mutation being associated with increased susceptibility to TPV, the I50V mutation was de-selected after virological failure of an Aptivus/r regimen. In the majority of cases de-selection of multiple PI mutations including the I50V mutation occurred. It appears likely therefore that upon virological failure, amplification of a pre-existing minority/archived virus population occurs. In at least some cases there is evidence that additional protease mutations were subsequently added over time into this genetic background; e.g. Patient B (L10V, V82T), Patient F (I13V), Patient G (V82T, I84V), Patient I (L33V).
Further sequence analysis may provide additional information on the influence of
minority species on the response to Aptivus/r therapy and the dynamics of the
de-selection of the I50V mutation during initial suppression and virological rebound. Of note, at least a two log (copies/mL) decrease in baseline viral load preceded virological rebound in the nine patients failing therapy (Figure 1). Limited activity of the background regimens of these heavily pretreated patients may well explain the lack of a durable virological response.
In summary, a clinical isolate harboring an I50V protease mutation would be expected to have increased susceptibility to TPV and the patient would be expected to have a better virological response to an Aptivus/r containing regimen compared to a CPI/r containing regimen. Upon virological failure of Aptivus/r, de-selection of the I50V mutation would be expected to occur.
References
Batchelor et al (2006) 4th Euro. HIV Drug Res. Workshop, Monte Carlo, Monaco.
Hall et al (2006) Wkshop. On Quant. Meth. for Res. on Antiviral Resistance. Boston, USA
Johnston et al (2005) Topics in HIV Medicine 13(4):125-131
Larder et al (2000) AIDS 14(13):1943-1948
Maguire et al (2002) Antimicrob. Agents & Chemother. 46:731-738
Mo et al (2005) Virology 79:3329-3338
Parkin et al (2003) AIDS 17:1077-1080
Parkin et al (2006) 13th CROI, Denver, USA. Abstract 637
Partaledis et al (1995) J.Virology 69:5228-5235
Sax et al (2005) 45th ICAAC, Washington, USA Abstract H-1060
Yates et al (2006) Antimicrob. Agents & Chemother. 50:1092-1095
Yoshimira et al (2002) J. Virology 76:1349-1358
|
| |
|
 |
 |
|
|