 |
 |
 |
| |
Initial Virologic Failure with HIV Drug Resistance and Impact of Resistance on Disease Progression and Death for Patients Beginning PI, NNRTI or PI+NNRTI Based Strategies: The FIRST Study
|
| |
| |
Reported by Jules Levin
XV Intl HIV Drug Resistance Workshop
June 13-17, 2006, Sitges, Spain
Michael J Kozal, MD1, Kathy Huppler Hullsiek, PhD2, Rodger D MacArthur, MD3, Grace Peng, MS2, Ying Xiang, MS2, John D Baxter, MD4 M Van den Berg-Wolf, MD5, Jonathan Uy, MD6, Edward E Telzak, MD7 and Richard M Novak, MD6
for the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA)
1. Yale University School of Medicine, New Haven, CT, USA 2. University of Minnesota, Minneapolis, MN, USA 3. Wayne State University, Detroit, MI, USA 4. Cooper Hospital/RWJ Medical School, Camden, NJ, USA 5. Temple University, Philadelphia, PA 6. University of Illinois, Chicago, IL, USA 7. Bronx Lebanon Hospital, Bronx, NY, USA.
Note from Jules Levin: My understanding is that 25% of those taking a PI used ritonavir boosting since the study started over 5 years ago. This analysis reports the resistance data from the FIRST Study and the response data will be reported at the international conference in Toronto in August. Neither this analysis nor the Swiss Study also reported at the Workshop and which also found benefit in terms of resistance compared to NNRTI regimens does not break down which specific PI drugs patients were taking.
OBJECTIVES
To compare the three initial randomized ART strategies of FIRST for the development of drug resistance at first virologic failure and cumulatively over time. (PI + 2 NRTIs, NNRTI + 2 NRTIs, PI + NNRTI + 1 oe 2 NRTIs).
To determine the impact of antiretroviral drug resistance on progression of disease (POD), defined as death or an AIDS event.
AUTHOR CONCLUSIONS
- Although the rate of 1st VF was highest in the PI strategy, the rate of 1st VF with resistance was only marginally higher in the PI compared to the NNRTI strategy.
- For treatment-naive patients on three ART strategies, the risk of having an AIDS event or death varied with the ability to achieve virologic suppression prior to virologic failure and with the class(es) of drug resistance that developed.
- Patients with PI resistance at first VF did not have an increased risk of progression of disease compared to those with no VF. Conversely, patients with NNRTI resistance were 4.4 times more likely to have progression of disease than those without VF.
- Patients with PI resistance at initial VF were more likely to achieve a viral load < 400 c/ml after VF than those with other resistance patterns.
- Considering the impact of resistance by class, NNRTI resistance was the strongest predictor of disease progression.
BACKGROUND
- The FIRST study was a randomized trial conducted by the CPCRA at 80 research sites in the USA. The study evaluated the long-term clinical, immunologic, and virologic effects of 3 initial ART strategies for ART naive patients:
a) PI strategy (PI plus 2 NRTIs)
b) NNRTI strategy (NNRTI plus 2 NRTIs)
c) 3-class strategy (PI+NNRTI + 1 or 2 NRTI).
- There were 2 major study objectives :
1) to compare the PI and NNRTI strategies for a composite primary endpoint of AIDS or death or (for those with a baseline CD4+ count ³200 cells) a follow-up CD4+ count of <200 cells.
2) to compare the 3-class strategy with the pooled results of the two 2-class strategies for the difference in CD4+ cell count after 32 months of follow-up.
- A total of 1397 participants were randomized to the FIRST study and were followed for a median of 5 years (range 44-78 months). The results of the FIRST study will be presented at the XVI International AIDS Conference, August 13-18, 2006, Toronto, Canada.
- Briefly, the PI and the NNRTI strategies did not differ for a composite outcome based on CD4 cell count decline, AIDS events, or death, although the NNRTI strategy had a superior virologic response compared to the PI strategy. The 3-class strategy was not superior to a 2-class strategy for immunologic or clinical outcomes.
METHODS
- HIV-1 RNA levels were obtained at months 1 and 4, and then every 4 months. Viral load (VL) measurements were determined using the Roche Ultrasensitive Amplicor assay (limit of detection <50 HIV RNA copies/mL).
- Virologic failure (VF) was defined as an HIV RNA level >1000 copies/mL at or after the 4-month visit.
- Genotypic resistance testing was performed at three possible time points: initial virologic failure and (for those with VL > 1000 copies/mL) at months 12 and 36.
- Resistance was defined as definite drug resistance by genotype, using the TRUGENE HIV-1 Genotyping Kit and OpenGene DNA Sequencing System, Bayer Diagnostics (Version 4.0) and CPCRA interpretive algorithm (v4.0), which presented drug susceptibilities for 16 drugs (7 NRTI, 3 NNRTI, and 6 PI).
- The presence of specific drug-resistance mutations was also considered, using the October 2004 IAS-USA tables.
Statistical Analysis: Strategies were compared (intent-to-treat) for first VF with definite drug resistance using chi-squared tests and time to event methods. For each strategy and for the entire cohort proportional hazards models with time-dependent covariates were used to determine the impact of resistance on AIDS or death events occurring after at least 4 months. Rates are per 100 person years.
RESULTS
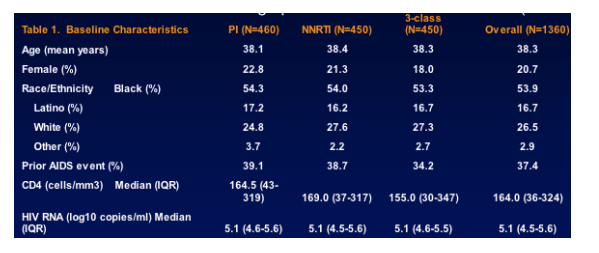
- Of the 1397 participants randomized, at least one VL measurement was available for 1360 (97%) participants at or after the 4-mo visit (minimum time point for determining VF): 460 in the PI arm, 450 in the NNRTI arm, and 450 in the 3-class arm. The 3 randomized strategies were well balanced in terms of demographic and baseline characteristics (Table 1).
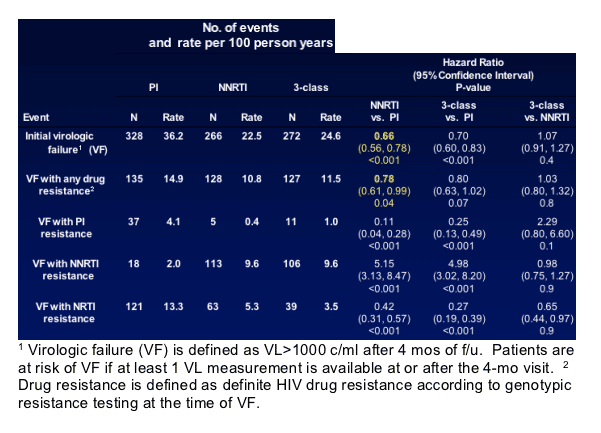
- Of the 866 participants who experienced VF, 328 (37.9%) were randomized to the PI strategy, 266 (30.7%) in the NNRTI strategy, and 272 (31.4%) in the 3-class strategy. Genotypic resistance test results were available for 858 (99%) of those with VF.
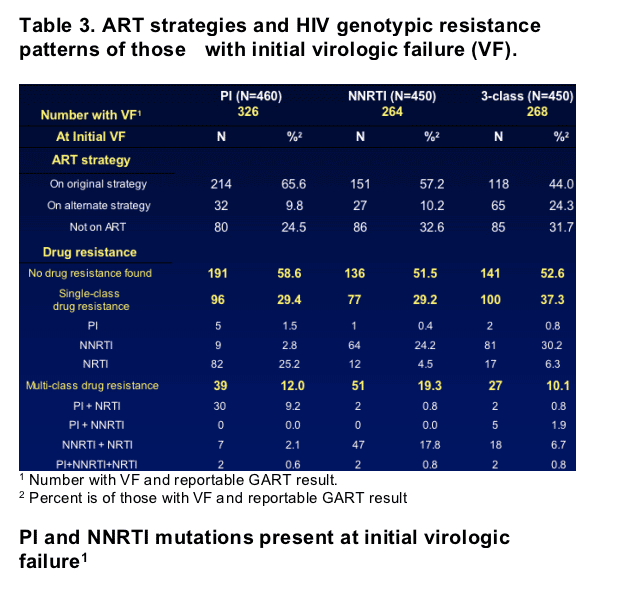
- Of the 858 with at least one resistance test, 316 (37%) had two resistance tests (at initial VF and either month 12 or 36), and 119 (14%) had three resistance tests (at VF mos 12 & 36).
- Of those with a resistance test at any time during follow-up, no major mutation was ever detected in 46%, 39%, and 40% of those randomized to the PI, NNRTI, and 3-class strategies, respectively, while multi-class mutations were detected in 26%, 34%, and 21%.
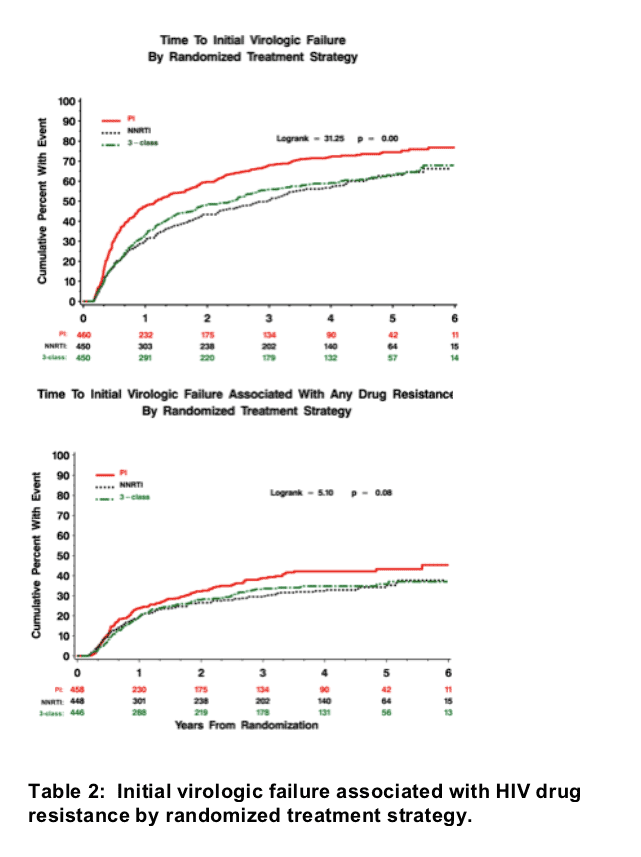
Figure 1. Time to first HIV RNA >1000 copies/mL.
The cumulative percent with an initial virologic failure was significantly higher in the PI strategy compared to the NNRTI and 3-class strategies (Figure 1 and Table 2).
Figure 2. Time to first HIV RNA >1000 associated with any drug resistance.
The cumulative percent with first virologic failure associated with any type of drug resistance was marginally higher in the PI strategy compared to NNRTI and 3-class strategies (Figure 2 and Table 2).
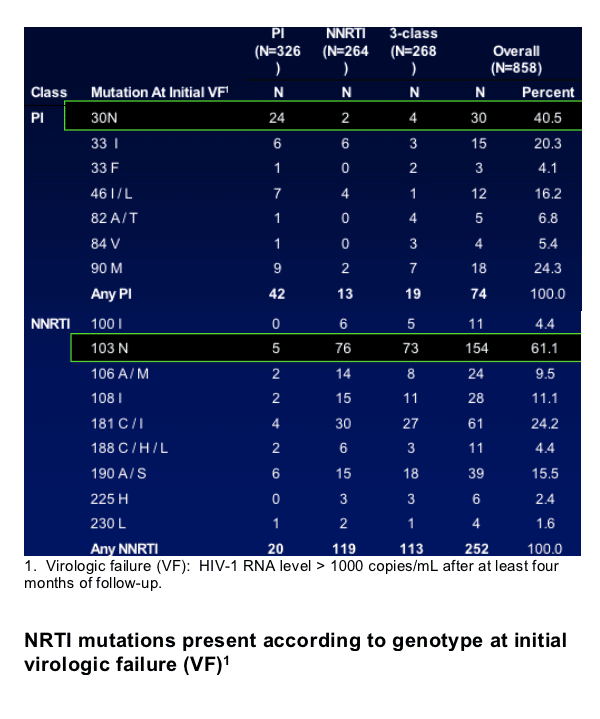
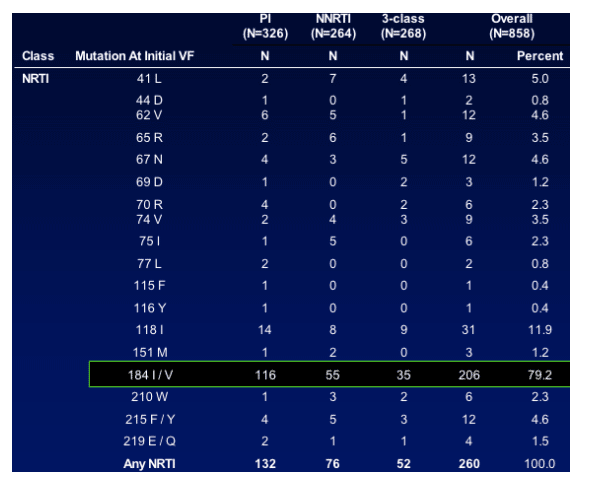
Results: Predicting HIV Progression of Disease
- There were1302 participants who were at risk for an AIDS or death event after the 4-mo visit, the earliest time point at which VF could be determined.
- In a model considering the impact of resistance at VF and the ability to achieve viral suppression (VL<400 copies/mL) prior to VF, there was significantly increased risk of AIDS or death compared to those with no VF for those failing : (a) with resistance and prior suppression (HR= 3.0, p<0.001); (b) with resistance and no prior suppression (HR= 4.6, p<0.001); (c) without resistance and with prior suppression (HR=2.9, p<0.001); and (d) without resistance and no prior suppression (HR= 6.5, p<0.001).
- To further investigate the impact of resistance at VF and the ability to achieve viral suppression on subsequent AIDS or death events, 5 mutually exclusive groups were formed according to the class(es) of resistance detected at VF: any PI resistance (N=52); only NNRTI resistance (N=146); only NRTI resistance (N=108); NNRTI and NRTI resistance (N=68); and no resistance (N=457).
- These 5 groups were compared for the risk of an AIDS or death event compared to those who did not experience VF (N=471). Cox regression models were fit separately for each treatment strategy and overall, adjusted for baseline variables (a prior AIDS event, CD4 cell count, HIV RNA level, and age) and time-updated factors (resistance group, missing HIV RNA level, and the ability to achieve viral suppression prior to VF). The numbers of participants with an event in each resistance group and the hazard ratios for an event (compared to those with no VF) are given in Tables 5 & 6.
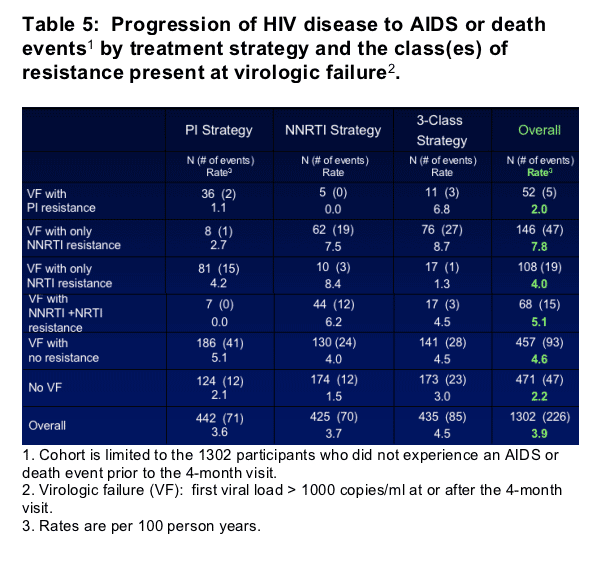
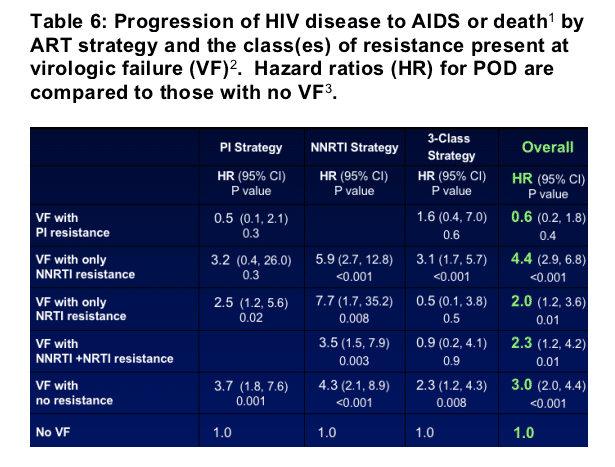
1 Cohort is limited to the 1302 participants who did not experience an AIDS or death event prior to the 4-month visit. 2 Virologic failure (VF): first viral load > 1000 copies/ml at or after the 4-month visit. 3 Hazard ratios and confidence intervals are estimated using Cox regression models fit separately for each treatment strategy and overall. All models are adjusted for baseline variables (prior AIDS, CD4 cell count, HIV RNA level, and age) and time-updated factors (the groups listed, missing HIV RNA levels, HIV RNA level < 400 copies/ml prior to VF).
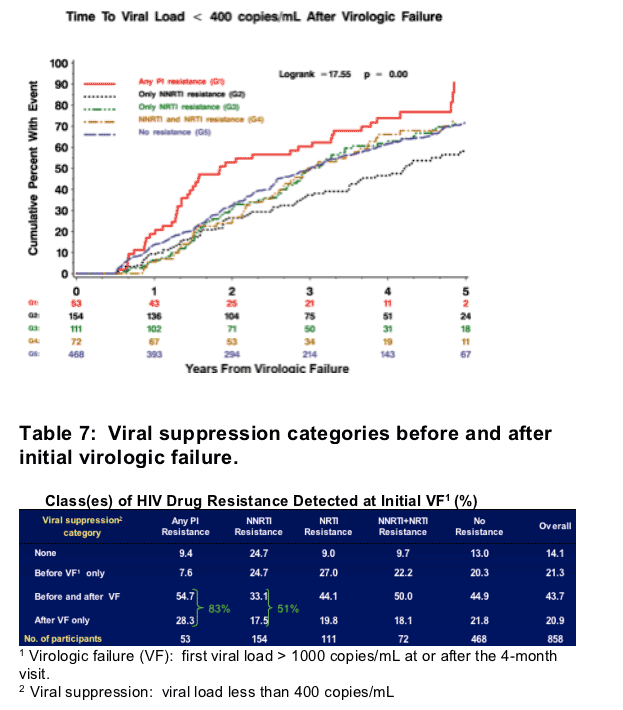
Figure 3. Time to viral load <400 copies/ml after initial virologic failure.
To explore why those with some resistance patterns have increased risk of an AIDS event or death after VF, we investigated which groups were able to achieve virologic suppression (VL < 400 copies/ml) after initial VF. Of those failing with PI and NNRTI resistance, 83% and 51% respectively went on to experience suppression (Table 7). From exploratory analyses, those failing with PI resistance were significantly more likely to achieve viral suppression after VF: hazard ratios and 95% CI
- 1.6 (1.2 -2.2) compared to those failing with NNRTI resistance
- 1.8 (1.3-2.6) compared to those failing with any other resistance
- 1.5 (1.1 -2.0) compared to those failing with no resistance
|
| |
|
 |
 |
|
|