 |
 |
 |
| |
Improvement in Insulin Sensitivity and Dyslipidemia in Protease Inhibitor-Treated Patients After Switch to Atazanavir/Ritonavir (ATV/RTV): A Prospective Study Using Hyperinsulinemic, Euglycemic Clamp Testing
|
| |
| |
Reported by Jules Levin
8th Intl Workshop on Adverse Drug Reactions and Lipodystrophy in HIV
San Francisco, Sept 24-26, 2006
Anthony J. Busti, Pharm.D.1,3, Roger Bedimo, M.D., M.S.2,3, David M. Margolis, M.D.4, Dana Hardin, M.D.5
Texas Tech University Health Sciences Center School of Pharmacy Dallas Fort Worth Regional Campus, Dallas, TX1; The University of Texas Southwestern Medical Center, Dallas, TX2;
Dallas Veteran's Affairs Medical Center, Dallas, TX3; University of North Carolina at Chapel Hill, Chapel Hill, NC4; Ohio State University, Columbus, OH5
The authors concluded:
-- In the first study using the gold-standard euglycemic clamp
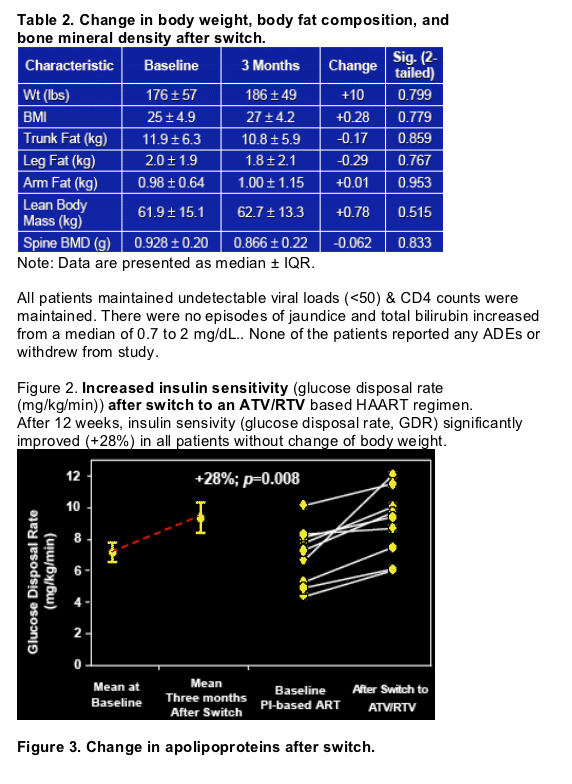
atazanavir/ritonavir improved PI-induced insulin resistance in dyslipidemic HIV+ patients on PI-based ART. (Insulin sensitivity [glucose disposal rate, GDR] significantly improved by 28% in all patients without change in body weight).
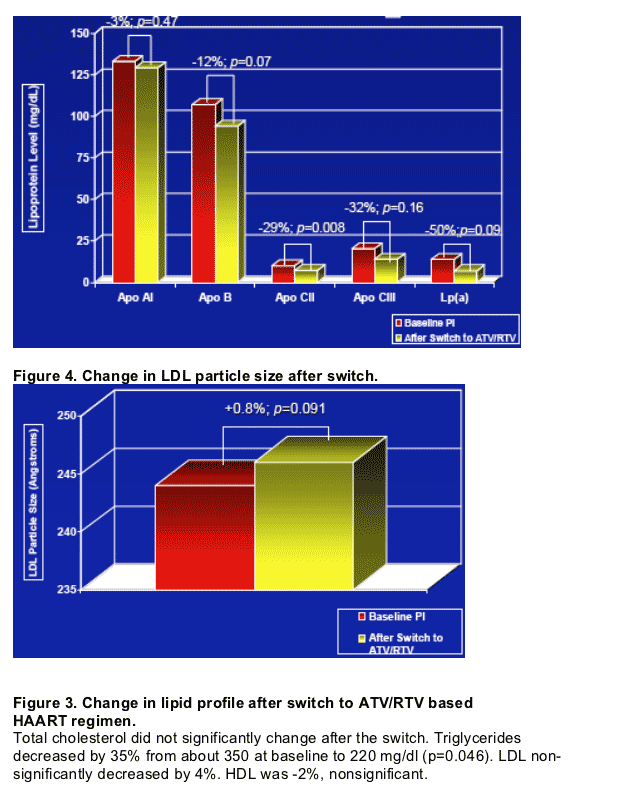
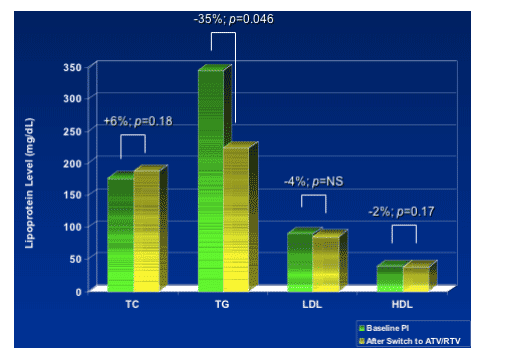
-- Switching to atazanavir/rtv based ART regimen resulted in improved atherogenic lipid profile.
-- These changes are not attributable to change in body weight and provide direct evidence for atazanavir's unique metabolic profile among the HIV protease inhibitors.
Background
Treatment of HIV with some protease inhibitors is associated with insulin resistance and triglyceride-rich dyslipidemia and fat redistribution. Atazanavir is a potent once daily PI with fewer metabolic abnormalities in HIV+ patients.
Approximately 47-54% of HIV patients on PI-based ART (excluding atazanavir) are known to have dyslipidemia and insulin resistance. 1
The prevalence of impaired glucose tolerance (IGT) in a cohort of PI-treated, lipodystrophic HIV patients was reported to be 35%. 2
The prevalence of diabetes is 4-fold higher in HIV patients who are treatment experienced compared to ART-naive. 3
".....The overall prevalence of diabetes mellitus in the DAD study was 2.5% and varied between regimens, from 1.1% in patients not currently receiving antiretroviral therapy to 4.3% in patients receiving PI and NNRTI. This is consistent with other studies which have shown an association between impaired glucose tolerance, diabetes mellitus and use of PI. The prevalence of diabetes in PI-treated HIV patients has been reported to be in the range of 2-8%, with the highest detection rate in studies based on performance of oral glucose tolerance testing. In the setting of our observational study, in which oral glucose tolerance testing is not mandated and the diagnosis will mainly rely on measuring repeated elevated fasting blood sugar, the prevalence of diabetes is likely to be underestimated...."
The estimated frequency of new onset diabetes on PI-based rgimens is 3-5%. 2
It is known that cardiovascular mortality increases with IGT and diabetes compared to non-diabetic patients. 4
PIs are known to decrease insulin sensitivity, decrease glucose disposal rate, and adipocyte glucose uptake after both single and repeated dosing. 5,6,7
Atazanavir's effects on insulin resistance and glucose uptake have been studied only as a single agent in healthy HIV-negative volunteers. 8,9
STUDY OBJECTIVES
Primary Objective:
- To determine the effects on insulin sensitivity using hyperinsulinemic, euglycemic clamp when switching from PI-based ART in patient with dyslipidemia to ATV/RTV-based ART.
Secondary Objectives:
- To determine changes in lipoprotein profiles (TC, TG, HDL, LDL, Non-HDL, Apo B, Apo CII, Apo CIII, LDL & HDL subtypes, and Lp(a)) and effect on atherogenic
dyslipidemia.
- To determine any changes in percent of body fat, lean body mass, and bone density by whole body DEXA scans.
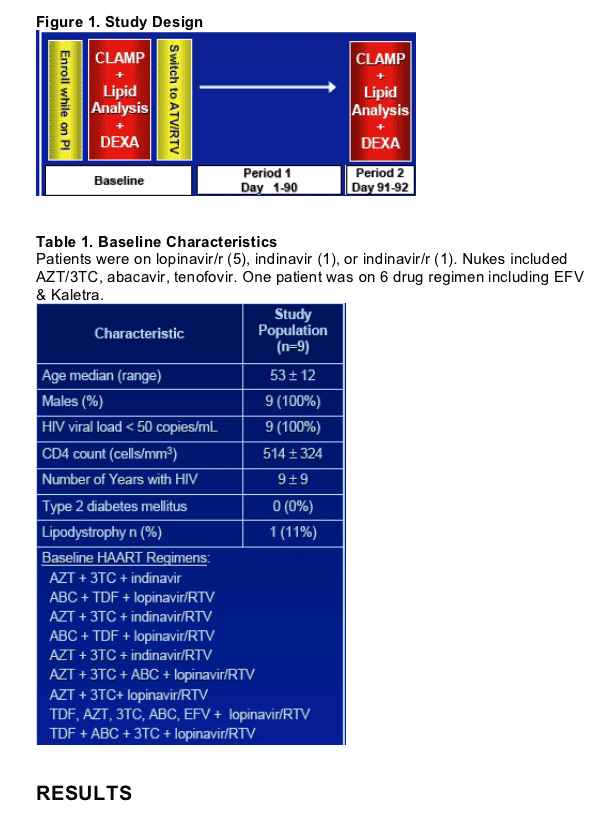
METHODS
Single-center, prospective study on 9 adult, male HIV+ patients with documented dyslipidemia while on a PI-based ART regimen. PI regimen was changed to ATV/RTV (300/100 mg once daily). Inclusion criteria were VL <50 c/ml, CD4 >200 cells, triglycerides >200 mg/dl.
Follow-up visits with fasting labs every month x 3 months.
Whole body insulin sensitivity was assessed during a 3-hr hyperinsulinemic euglycaemic clamp (200 mU/m2/min) and whole body composition was measured by (whole-body DEXA) at baseline and after 12 weeks of ATV/RTV:
Hyperinsulinemic, euglycemic clamp:
- Standard, three-step clamp set-up to determine peripheral insulin sensitivity at baseline and at 3 months.
Lipoprotein analysis:
- Assessed in fasting state by standard gradient gel electrophoresis at baseline and at 3 months.
Fat distribution:
-Whole body DEXA scans at baseline and 3 months.
Data analysis:
- All continuous data were analyzed using Wilcoxon Signed Ranks Test and presented as median differences and/or ± IQR.
References
1.USDHHS Panel on Antiretroviral Guidelines for Adolescents. May 4, 2006.
2. Hadigan G et al. CID 2001;32:130-9.
3. Friis Moller N et al. AIDS 2003;17:1179-93.
4. Eschwege E et al. Horm Metab Res Suppl 1985
5. Noor M et al. AIDS 2001;15:F11-8.
6. Lee et al. AIDS 2004:18:641-9.
7. Noor M et al. AIDS 2002;16:F1-8.
8. Noor M et al. AIDS 2004;18:2137-44.
9. Noor M et al. AIDS 2006;20:1813-21.
|
| |
|
 |
 |
|
|