 |
 |
 |
| |
Significant Improvements in Self-Reported Gastrointestinal Tolerability, Quality of Life, Patient Satisfaction, and Adherence with Lopinavir/ritonavir After Switching from BID Soft-Gel Capsules (SGC) to BID Tablets
|
| |
| |
Reported by Jules Levin
8th Intl Workshop on Adverse Drug Reactions & Lipodystrophy in HIV
San Francisco, Sept 24-26, 2006
S Schrader1, SK Chuck2, LW Rahn2, KG Emrich2, PS Parekh2,
1The Schrader Clinic, Houston, Tx, USA, 2Abbott Laboratories, Abbott Park, IL, USA; poster 81
AUTHOR CONCLUSIONS
In this US survey of 332 HIV-infected patients, significant improvements were reported by patients switching from LPV/r SGC dosed BID to Tablets dosed BID.
-- 82% of respondents reported no or improved diarrhea
-- 12% more respondents reported no or rare bloating, pain, gas in stomach
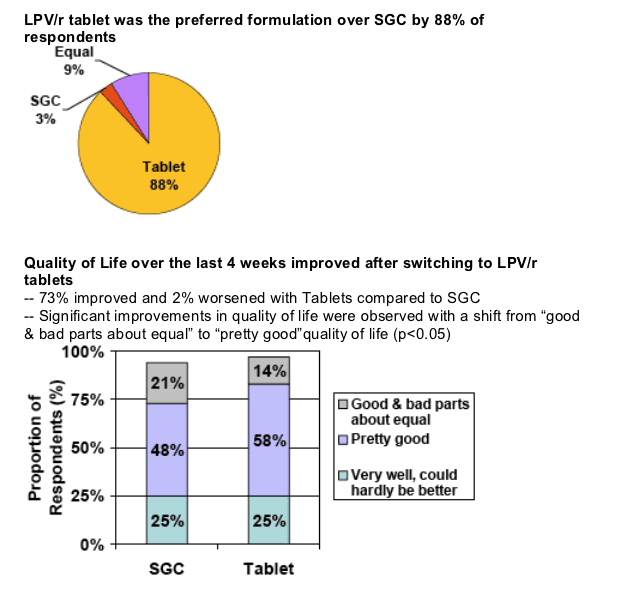
-- Significant improvements in satisfaction, as well as overall tolerability for 20% of respondents
-- Adherence improved from 91% to 95%, with 7% more respondents reporting no missed doses
The LPV/r Tablet benefit most frequently cited by respondents were
Don't have to refrigerate (67%), fewer pills (61%), don't have to take with food (41%).
These results suggest that LPV/r Tablets dosed BID provides multiple benefits to HIV patients relative to SGC. Additional study to further define the tolerability profile of LPV/r Tablets is warranted.
BACKGROUND
Kaletra (lopinavir/ritonavir, LPV/r) Tablets were FDA approved in October 2005.
Short-term results in HIV-negative, healthy volunteers suggest improved tolerability, but data in HIV-infected patients have not been previously reported. [Klein C, et al. EACS, 2005, PE4.3/2]
Features of LPV/r Tablets compared to Soft Gel Capsule (SGC)
[Kaletra US Prescribing Info. 10/05]
-- Based on novel Melt-Extrusiontechnology
-- No oleic acid or sorbitol
-- Contains 200 mg of lopinavir and 50 mg of ritonavir
-- Daily pill count decreased from 6 to 4 for same daily dose of 800/200 mg
-- No refrigeration
-- No need for dosing with food
-- Less pharmacokinetic variability
STUDY OBJECTIVES
To assess patient self-reported differences between LPV/r SGC and Tablet formulations when dosed twice daily (BID)
Satisfaction
Tolerability
-Overall
-Frequency and severity of select adverse effects
-Diarrhea & antidiarrheal use
Adherence
-Missed doses, fewer pills, food requirement with SGC
-Reasons for missed doses
Benefits
Quality of life
To determine patient preference between LPV/r SGC and Tablet formulations when dosed BID.
METHODS - Study Design
Self-reported, anonymous, multiple-choice survey in English & Spanish
Addresses satisfaction, overall tolerability, adverse effects, adherence, perceived benefits, formulation preference, and quality of life
SGC and Tablet surveys had identical questions with 4 additionalcomparative questions (SGC vs. Tablets) in the Tablet survey
Respondents were asked to think back over the last 4 weeks and indicate in a typical week the frequency & severity of side effects
Adherence was reported on based on the last week of dosing
Questions written at grade 6 level
METHODS - Survey Distribution
52 out of 65 US physicians contacted distributed surveys to patients; a small payment to physicians were made for efforts related to distribution and handling of surveys with a maximum of 25 patients per site allowed; patients received no compensation
Physicians provided surveys to the patients while on LPV/r SGC and LPV/r Tablets dosed at 400/100mg BID after a minimum of 4 weeks on each formulation.
Patients completed the surveys in waiting area at their routine scheduled visits.
Patient privacy was maintained by having patients seal completedsurveys into envelopes prior to providing survey to clinic staff for mailing to research company managing the project.
October 2005 through May 2006
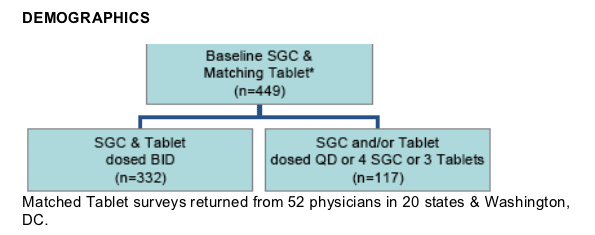
Matched Tablet surveys returned from 52 physicians in 20 states & Washington, DC.
332 respondents were mostly males (85%) with diverse ethnicity.
Majority of respondents were >35 years old:
- 46% were > 45 years old
- 41% were 35-44 years old
- 13% were < 35 years old
Duration of antiretroviral therapy:
59% at least 5 years
31% 1 to 5 years
10% < 1 year
Duration of LPV/r therapy:
82% of LPV/r SGC experience was > 1 year
89% of LPV/r Tablet experience was < 3 months
Black non-hispanic: 37%
Hispanic: 19%
White non-hispanic: 41%
RESULTS
84% of respondents indicated "pretty good"or "great"tolerability after switching to LPV/r Tablets dosed BID; 21% more than with SGC (p<0.05)
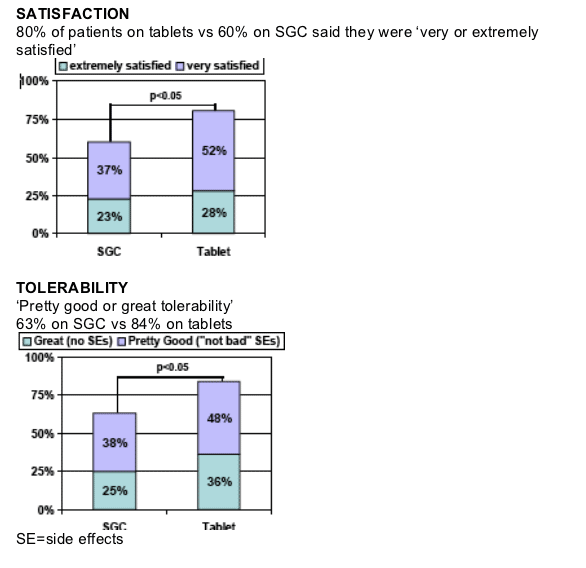
Respondents had a significant improvement in diarrhea after switching to LPV/r tablets
- 82% reported no diarrhea or improvement
- 21% more respondents indicated no or rare diarrhea (p<0.05)
- Only 3% reported "severe"diarrhea (vs. 12% for SGC, p<0.05)
- Antidiarrheal use of 3+ times per week was reduced by half (p<0.05)
- 76% of respondents reported no or rare antidiarrheal use (p<0.05)
Significantly fewer respondents reported bloating, pain, or gas in stomach and those who did had diminished frequency on LPV/r tablets
- 12% more respondents indicated no or rare occurrence (p<0.05)
- Only 5% reported "severe"episodes (vs. 8% for SCG, p<0.10)
Self-reported adherence based on missed doses significantly improved after switching to LPV/r tablets
Mean number of missed doses per wk decreased (from 1.25 to 0.71,p<0.05)
-this is equivalent to adherence improving from 91% to 95%
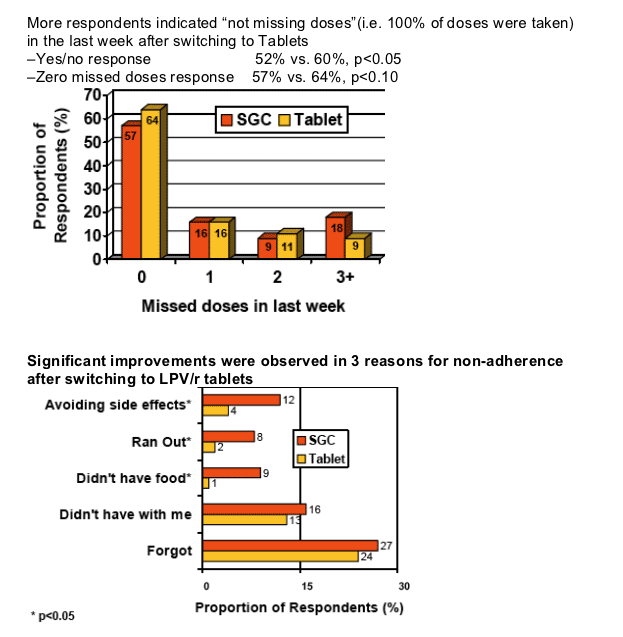
LPV/r tablet benefits cited by respondents related to refrigeration, pill count, and food requirement
Respondents cited the following as benefits they "liked"
- Don't have to refrigerate (67%)
- Fewer pills (61%)
- Don't have to take with food (41%)
41% of respondents cited the lack of dietary restriction as a benefit. This may be explained by the similar level of non-adherence to LPV/r SGC's food requirement.
- 44% of respondents indicated at least 1 dose of LPV/r SGC was taken without food in the last week
- An average of 16% of LPV/r SGC doses were taken without food
|
| |
|
 |
 |
|
|