 |
 |
 |
| |
Improved Triglycerides and Insulin Sensitivity with 3 mos of Acipimox in HIV-infected Patients with Hypertriglyceridemia
|
| |
| |
Reported by Jules Levin
8th Intl Workshop on Adverse Drug Reactions and Lipodystrophy in HIV
San Francisco, Sept 24-26, 2006
C. Hadigan, J. Liebau, M. Torriani, R. Andersen, S. Grinspoon
Harvard Medical School, Boston MA
NIH, NIAID, Bethesda MD
AUTHOR CONCLUSIONS:
Acipimox resulted in reduced triglycerides and improvements in insulin sensitivity over 3 mos in HIV+ patients with hyperlipidemia and lipodystrophy
20% reduction in median Tg is similar to 25% reduction at 24 wks (Dube et al, ADRL 2005 Dublin) with extended release niacin
At 14 wks Gerber et al (CID 2004) showed a 34% reduced median Tg, but also significant increases in HOMA with extended release niacin
Caveats - small study, relatively short duration, TID regimen
Remaining Questions
-- Additive benefits of using acipimox in patients with suboptimal response to statins and/or fibrates
-- Potential benefits of longer duration therapy on lipids and over all metabolic profile
-- Possibility of effects on body fat depots through chronic blockade of lipolysis
RESULTS:
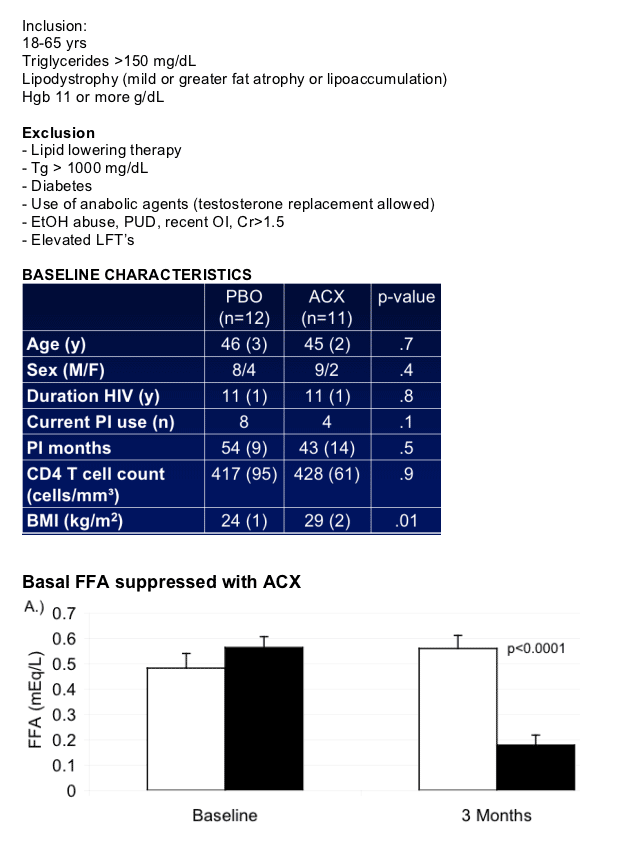
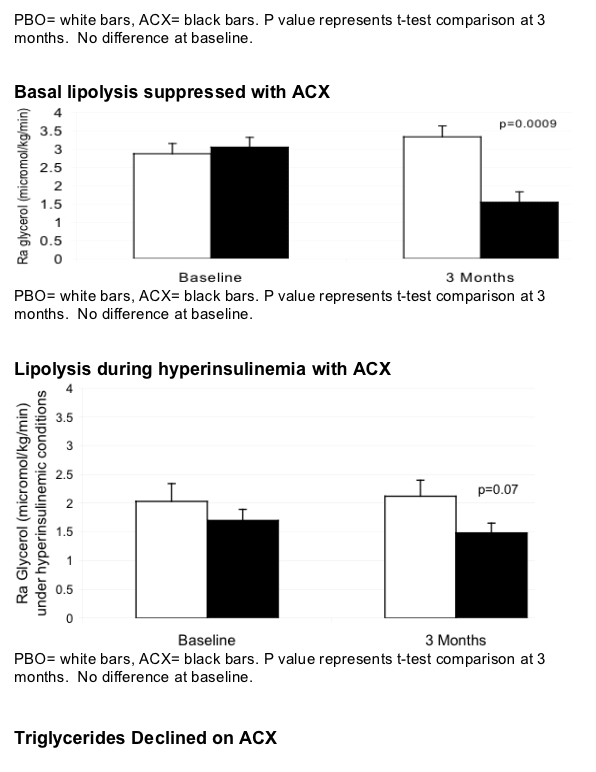
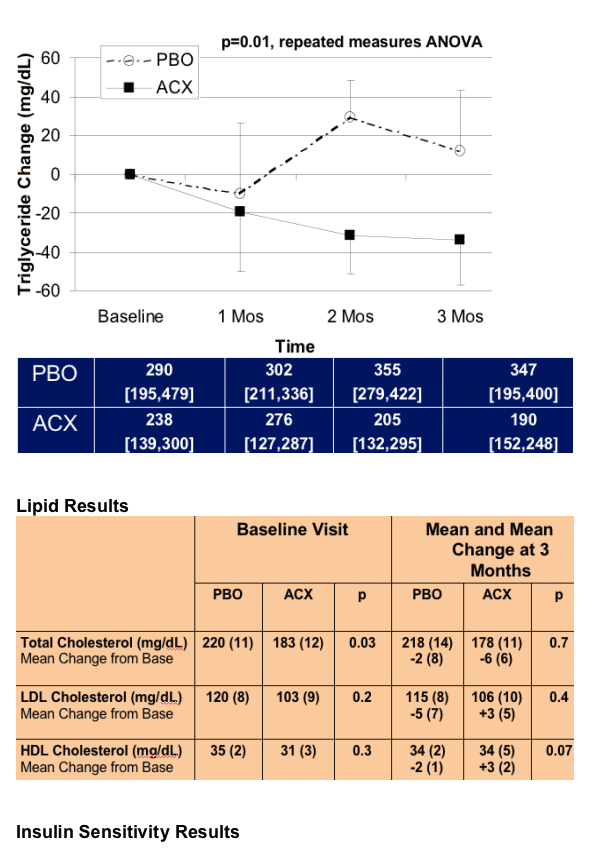
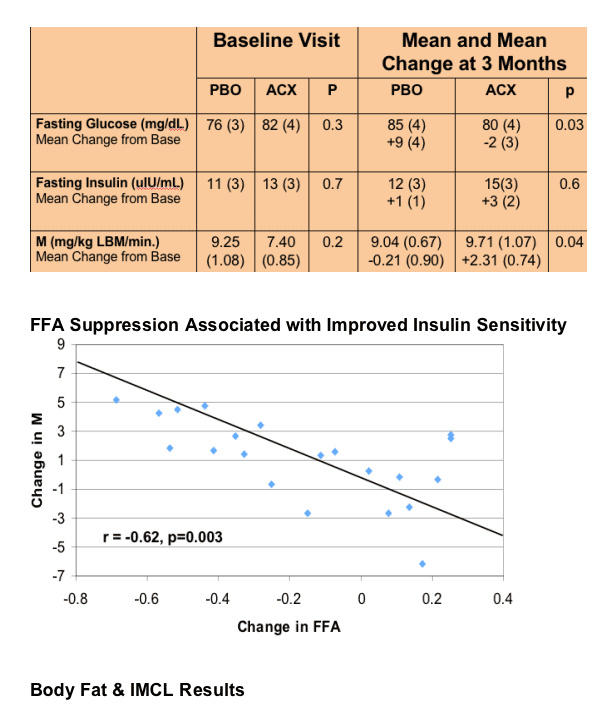
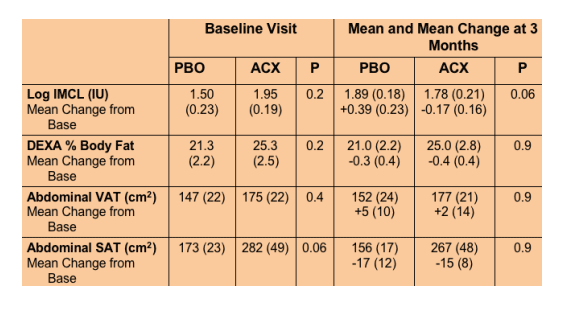
Acipomax resulted in significant sustained reduction in FFA (mean change -0.38 [0.06] versus 0.08 [0.06] mEq/l with placebo, p<0.0001), decreased rates of lipolysis (p<0.0001), and a 34 mg/dl mean reduction in triglyceride concentration at 3 months (MANOVA compared to placebo, p=0.01). In addition, acipomax administration was associated with improved insulin sensitivity in hyperinsulinemic euglycaemic clamp testing (mean change in M [mg glucose/kg lean body mass/min] acipomax +2.31 [0.74] versus placebo -0.21 [0.90], p=0.04). Improvements in glucose disposal were significantly correlated with reductions in FFA (r= -0.68, p=0.0007) and lipolysis (r= -0.62, p=0.003). There was also a trend towards decreased intramyocellular lipid content after acipomax administration (p=0.06).
BACKGROUND
Hypertriglyceridemia is common in HIV, particularly in the setting of antiretroviral therapy.
Hadigan et al. CID, 2001,Mulligan et al. J AIDS 2000
Difficult to achieve target lipid goals with HMG-coA reductase inhibitors and fibrates in HIV
Aberg et al. AIDS Res Hum Retroviruses, 2005
Increased lipolysis and elevated free fatty acids (FFA) in HIV may contribute to hyperlipidemia and insulin resistance
Vigouroux et al. Diabetes Metab 1999, Meininger et al. Metabolism 2002, Reeds et al. Am J Phys Endo Metab 2003.
Role of Free Fatty Acids (FFA):
-- Strong correlations between FFA and insulin sensitivity in HIV+ men and women with lipodystrophy (n=66)
Meininger et al (Metabolism 2002)
-- FFA correlated positively with triglyceride levels (r=0.33, p=0.005)
Acipimox is a nicotinic acid analogue and a potent inhibitor of lipolysis used to treat hyperlipidemia
Acipimox was superior to pravastatin in reducing TG and increasing HDL in non-HIV hyperlipidemia
Fogari et al. Int J Clin Pharm Ther 1997
Acute suppression of FFA with acipimox improves insulin sensitivity in HIV-associated lipodystrophy:
-- HIV+ men (n=7), on PI-containing HAART, with increased WHR, hyperinsulinemia
Hadigan et al. Am J Clin Nutr 2003
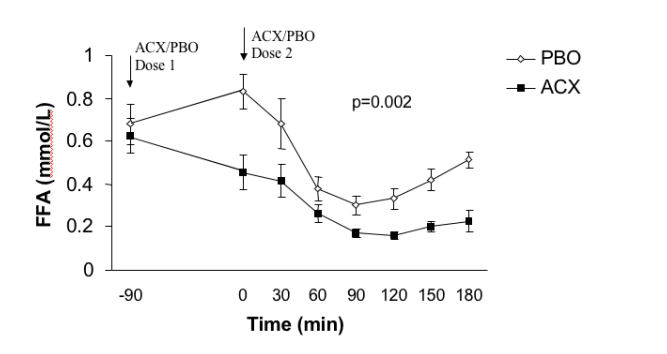
FFA area under the curve (AUC) reduction following Acipimox vs. Placebo
Hadigan et al. Am J Clin Nutr 2003
STUDY HYPOTHESIS
Chronic administration of Acipimox will result in improved triglyceride levels and insulin sensitivity among HIV-infected men and women with hypertriglyceridemia and lipodystrophy.
STUDY DESIGN
3-month randomized, double-blind, placebo controlled trial of Acipimox (250 mg TID)
Outcome variables
Primary: Triglyceride level
Secondary: Insulin sensitivity
Subjects completed a detailed metabolic evaluation at baseline and 3 mos following randomization:
-- Fasting lipids
-- Hyperinsulinemic euglycemic clamp
--Stable isotope tracer infusion with glycerol to assess lipolysis
-- Body composition
DXA
Cross-sectional Ab CT
IMCL of calf muscle
Tolerability & Compliance
One subject randomized to placebo was d/c'd from the study at 2 mos due to increased Cr > 1.5
2 subjects c/o mild itching on initiation of ACX which resolved within 3 weeks
1 subject experienced a single episode of flushing
Compliance by pill count was 92% in PBO and 90% in ACX
|
| |
|
 |
 |
|
|