 |
 |
 |
| |
Risk Factors for Bone Loss in HIV Appear to be Associated with Weight Loss and Additional Risk Factors Found in General Population
|
| |
| |
Lipodystrophy Workshop Oct 2006
ABSTRACT 12
Antiviral Therapy 2006; 11:L9
Prevalence of secondary causes of osteoporosis among HIV infected individuals
G Guaraldi1, G Orlando1, N Squillace1, V Rochira2,
B Madeo2, L Zirilli 2, C Diazzi 2, G Caffagni 2,
E Baraldi3, GC Carani2, R Esposito1 and P Tebas4
1Department of Medicine and Medical Specialties, Clinics of
Infective Diseases, University of Modena and Reggio Emilia,
Modena, Italy; 2Department of Medicine, Endocrinology and
Metabolism, Geriatrics, University of Modena and Reggio Emilia,
Modena, Italy; 3Laboratory of Endocrinology, Policlinico of
Modena, Italy; 4University of Pennsylvania, PA, USA
Introduction: Patients with HIV infection have a high prevalence of osteopenia/osteoporosis. The frequency of secondary causes of osteoporosis and the extent of the work up needed to exclude them in this population is unknown.
Objectives: Retrospective study evaluating the frequency of secondary causes of osteoporosis among HIV infected individuals in a large HIV metabolic clinic in Modena, Italy.
Methods: 1,199 consecutive HIV positive individuals referred for consultation to the metabolic clinic of the University of Modena were induced. The workup included whole body and localized DXA, complete blood count, chemistry profile, serum calcium, PTH, TSH and vitamin D, total and free testosterone for
males. DXA and metabolic determinations were done in a single lab. Standard definitions were used for the underlying diagnosis.
Results: The median age was 42. Patients were 62% males, 48% smokers, 68% with low physical activity, 1.2% heavy ETOH users, 42% HCV co-infected and 9% HBsAg (+). Median current and nadir CD4 497 and 159 cells/mm3 respectively. 76% were on ART (45% PI based) and 82% of the treated had HIV RNA <400 copies/ml.
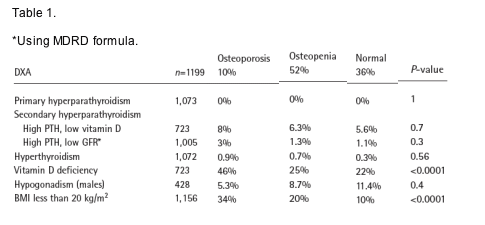
52% and 10% of the patients were osteopenic or osteoporotic in the lumbar spine or the hip according to WHO definitions. The frequency of secondary osteoporosis is summarized in Table 1.
Conclusions: Although osteopenia/osteoporosis is very frequent among HIV infected individuals, secondary osteoporosis is relatively rare. Vitamin D deficiency (and secondary hyperparthyroidsm associated with it) is frequent
in this Italian population and might be a contributor to this problem. The work up for secondary osteoporosis among HIV infected patients should include vitamin D levels, an assessment of renal function and total testosterone determinations. A more extensive work up is rarely useful and it should be reserved for the most severe cases.
ABSTRACT 104
HIV proteins suppress osteoblast function via modulation of RUNX-2 and PPAR_ transcription factor activity
EJ Cotter, N Chew, WG Powderly and PP Doran
UCD School of Medicine and Medical Sciences, Mater Hospital,
Dublin, Ireland
Aims: Decreased bone mineral density (BMD) has been associated with HIV infection, however the exact molecular mechanism of decreased BMD remains to be elucidated. RUNX-2 is a transcription factor that drives the development and maintenance of an osetoblastic phenotype in mesenchymal stem cells (MSCs) and osteoblasts (OBs), while PPAR_ is associated with pro-adipogenic development in MSCs. In this study we have examined in vitro the effect of exposure to HIV proteins on osteoblast function and on the expression and activity of PPAR_ and RUNX-2.
Methods: An immature human osteoblast (hOB) cell line (PromoCell) was cultured and treated with HIV p55-gag, HIV gp120 and HTLV env (100 ng/ml, 24 h) (NIH Aids Reagent Program). HTLV-1 env, was used to control for
non-specific viral effects. Cells were analysed for calcium deposition and alkaline phosphotase activity (ALP) using quantitative alizarin red staining and p-NNP assay respectively. Real-Time PCR with gene specific primers was used to analyse mRNA levels of RUNX-2, and PPAR_. RUNX-2 and PPAR_ activity were assessed using a commercially available assay (Active Motif).
Results: Exposure of hOBs to p55-gag and gp120 significantly altered osteoblast phenotype, as evidenced by reduced calcium deposition of hOBs by 30 ±10% and 29±8% and a reduction in ALP activity (11 ±5% and 19±0.1% respectively P=0.05). Both p55-gag and gp120 significantly reduced RUNX-2 mRNA levels (60 ±14% and 35% respectively P=0.05), however no significant changes in PPAR_ mRNA levels were observed. The activity of RUNX-2 was reduced by both p55-gag and gp120 (38±5% and 25 ±0.8% respectively P=0.05), while gp120 significantly increased PPAR_ activity (179 ±50%, P=0.05).
Conclusion: Our findings suggest that exposure to HIV proteins decreases bone formation in osteoblasts and this change in phenotype is paralleled by alterations in RUNX-2 and PPAR_ transcription factor activity in osteoblasts. Alterations of these activities may provide a molecular mechanism that contributes to HIV-associated reduction in BMD.
|
| |
|
 |
 |
|
|