 |
 |
 |
| |
Four-Year Entecavir Treatment in Nucleoside-Naive, HBeAg(+) Patients: Results from Studies ETV-022 and -901
|
| |
| |
Reported by Jules Levin
AASLD, Nov 2-6, 2007, Boston, MA
S Han1, TT Chang2, YC Chao3, SK Yoon4, RG Gish5, H Cheinquer6, FJ Carrilho7, H Zhang8, H Brett Smith8, R Hindes8
1Division of Digestive Disease, UCLA School of Medicine, Los Angeles, California, USA; 2National Cheng Kung University Medical College, Tainan, Taiwan; 3Tri-Service General Hospital, Taipei, Taiwan; 4Kangnam St. Mary's Hospital, Seoul, Korea; 5California Pacifi c Medical Center, San Francisco, California, USA; 6Universidade Federal Do Rio Grande Do Sul, Porto Alegre,
Brazil; 7Department of Gastroenterology, University of São Paulo School of Medicine, Sao Paulo, Brazil; 8Bristol-Myers Squibb, Wallingford, CT, USA
AUTHOR CONCLUSIONS
91% of nucleoside-naive HBeAg(+) patients who received continuous treatment with entecavir during 4 years had undetectable HBV DNA (<300 copies/mL by PCR) at Week 192
Continuous treatment during 4 years also resulted in maintenance of ALT normalization: 86% at Week 192
Treatment during years 3 and 4 resulted in additional patients achieving HBeAg loss and HBeAg seroconversion
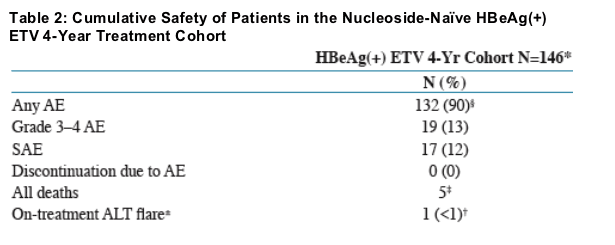
As previously reported, only one patient in this cohort developed resistance to entecavir1
Safety profile remained consistent with the previously reported experience
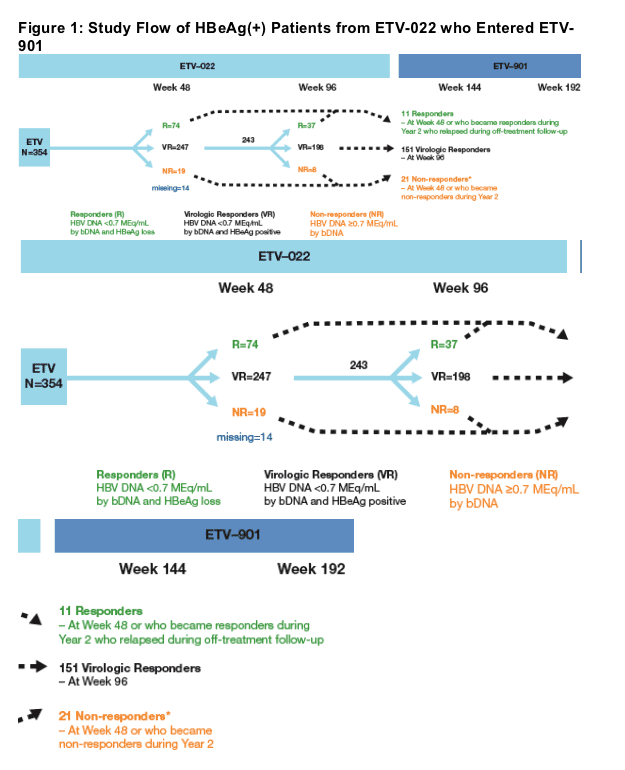
*Please note Poster 997 is Also Being Presented at AASLD Entitled 'Long-Term Follow-Up of Entecavir-Treated Protocol-Defined Non-Responders Rollover Study ETV-901.'
Introduction
The goal of chronic hepatitis B virus (HBV) treatment is to achieve sustained suppression of HBV replication and remission of liver disease
Entecavir 0.5 mg demonstrated superior virologic, histologic and biochemical activity compared to lamivudine 100 mg in study ETV-022 with minimal resistance
One hundred and eighty-three entecavir-treated patients from ETV-022 enrolled in rollover study ETV-901 (1 mg)
We present long-term efficacy, safety and resistance data of patients who received 4 years of continuous therapy with entecavir
Methods: Study Population
Study Population
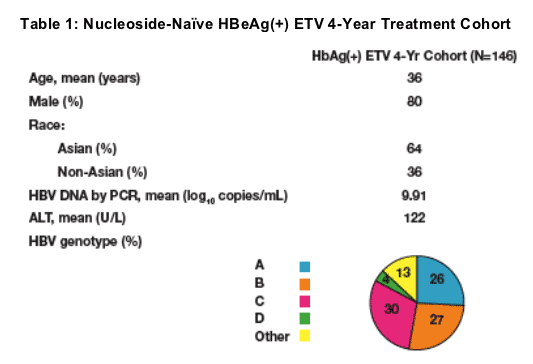
The Nucleoside-Naive HBeAg(+) ETV 4 Year Treatment Cohort (HBeAg(+) ETV 4 yr cohort) evaluated here consisted of patients who:
-- were initially treated with entecavir in ETV-022
-- subsequently enrolled in ETV-901 with ≦35 day gap in treatment between ETV-022 and ETV-901
This analysis cohort was defined without regard to:
-- treatment response at end of dosing in ETV-022
-- HBV DNA, ALT measurements or HBV serology at the start of dosing in ETV-901
Initially, due to ongoing blinding of studies ETV-022 and ETV-027, patients enrolling ETV-901 received a combination of ETV 1 mg and LVD 100 mg daily. Subsequently the protocol was amended for patients to receive monotherapy with ETV 1 mg daily
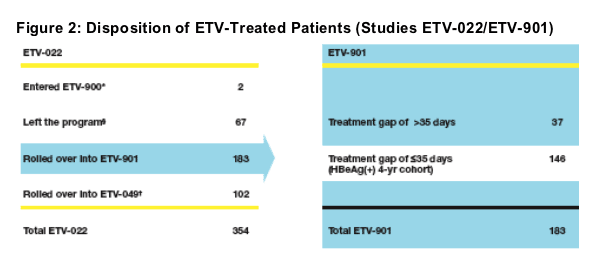
*ETV-900: Entecavir for patients with chronic hepatitis B infection: an early access program
Left the program: Did not enter a rollover trial (ETV-900 or ETV-901) or enroll in ETV 049 after completing study ETV-022
ETV-049: Long-term off-treatment assessment of treatment outcomes with entecavir and lamivudine for chronic hepatitis B infection in patients who have enrolled in phase III entecavir trials
Efficacy, Safety & Resistance Analyses
Proportions of patients with HBV DNA <300 copies/mL (measured by PCR), ALT ≦1 x ULN, HBeAg loss and HBeAg seroconversion among patients with available samples at Week 192
Paired samples from all patients with HBV DNA levels ≥300 copies/mL at Weeks 48, 96, 144, 192 or end of dosing were genotyped, and phenotype determined for all novel emerging substitutions
All patients experiencing a virologic breakthrough (≥1 log10 increase from nadir) were also phenotyped even if they did not have emerging substitutions
Safety was evaluated throughout the treatment period
Results
Undetectable HBV DNA
33 patients discontinued study therapy prior to Week 192; 24 (73%) had undetectable HBV DNA on their last PCR measurement
5 patients who remained on treatment at Week 192 had missing PCR values.
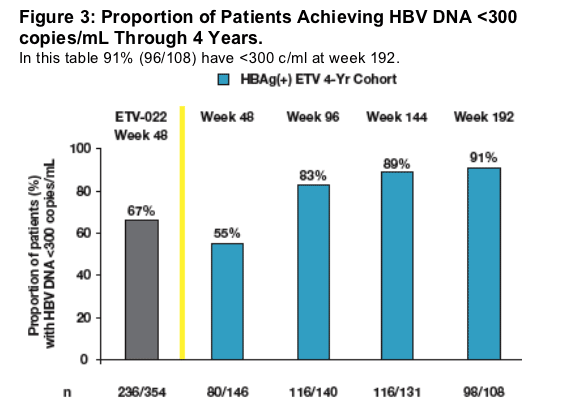
Results at Week 48 were consistent between the Nucleoside- Naive HBeAg(+) ETV 4 Year Treatment Cohort (55%) and the overall ETV-022 population (67%)
Treatment in Year 2 resulted in increasing proportions of patients achieving undetectable HBV DNA by PCR (<300 copies/mL)
Continuous treatment through Years 3 and 4 resulted in high proportions of patients maintaining undetectable HBV DNA
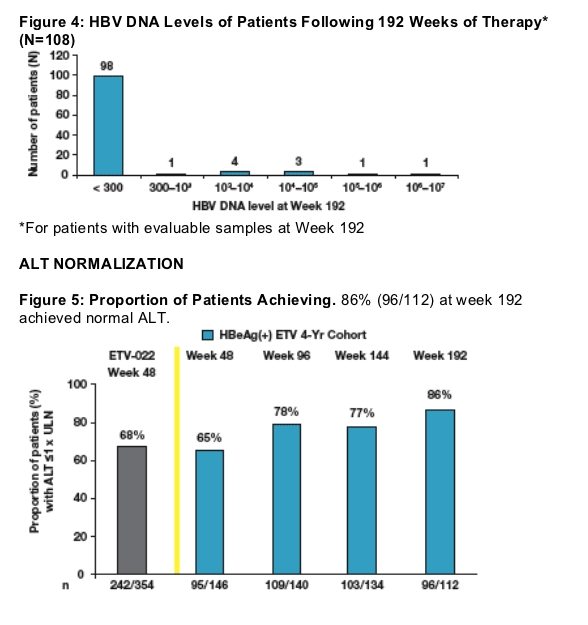
Results at Week 48 were consistent between the Nucleoside-Naive HBeAg(+) ETV 4-Year Cohort (65%) and the overall ETV-022 population (68%)
Treatment in Year 2 resulted in increasing proportions of patients achieving ALT normalization
Continuous treatment through Years 3 and 4 resulted in maintenance of ALT normalization
HBeAg loss and HBeAg seroconversion
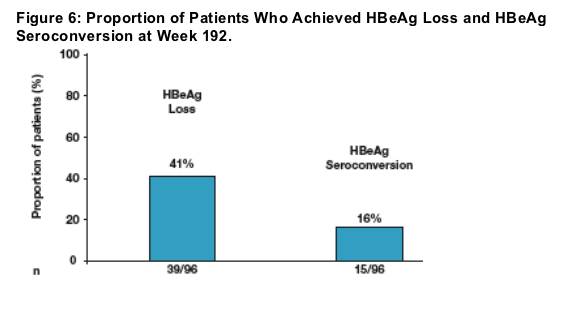
Serology tests within ETV-901 were performed at local laboratories
Due to protocol-def ned patient management criteria in ETV-022, most patients
who achieved HBeAg loss in Years 1 and 2 discontinued therapy and
were not included in the Nucleoside-Naive HBeAg(+) ETV 4-Year Cohort
Continuous treatment in Years 3 and 4 resulted in an additional 41% and 16% of patients achieving HBeAg loss and HBeAg seroconversion respectively
Proportions of patients achieving HBeAg loss or HBeAg seroconversion in this cohort represent additional patients from those previously reported in ETV-022 with 31% cumulative confirmed HBeAg seroconversion
Resistance Results
A total of 108/146 patients in the HBeAg(+) ETV 4 yr Cohort had HBV DNA measurements by PCR at Week 192:
-- 10 of these subjects had HBV DNA >300 copies/mL at Week 192
-- Genotypic testing showed that 1 out of the 10 subjects had simultaneous appearance of L180M & M204V (LVDr) and S202G (ETVr) substitutions at Week 1391. This patient had a virologic breakthrough at Week 148.
Thirty-three patients in the HBeAg(+) ETV 4-Year Treatment Cohort discontinued treatment prior to Week 192:
-- 9 of these subjects had HBV DNA >300 copies/mL at the last on treatment measurement. Genotypic analysis of these 9 subjects showed that none had evidence of genotypic ETVr
*Duration of treatment in ETV-901: 14 patients received ETV monotherapy only: mean 193 Wks (median 196 Wks); 132 patients received ETV and LVD combination therapy for a mean 26 Wks (median 24 Wks) followed by ETV monotherapy for a mean of 194 Wks (median 221 Wks)
Most common AEs, occurring in ≥10% of pts: Upper respiratory tract infection (31%), headache (21%), cough (17%), diarrhea (16%), influenza (17%),
nasopharyngitis (16%), pyrexia (12%), upper abdominal pain (10%)
Causes of death were: liver failure (1 patient); motorbike accident (1 patient); car accident (2 patients); unknown (1 patient). No deaths were attributed to study therapy by the investigator
On-treatment fl are and during follow-up: ± ALT fl are = ALT >2 Baseline ALT and >10 x ULN.
Reference
1. Colonno R, et al. Four Year Assessment of ETV Resistance in Nucleoside-Naive and
Lamivudine Refractory Patients. J Hepatol. 2007; 46 (Sup1), S294
|
| |
|
 |
 |
|
|