 |
 |
 |
| |
A Randomized, Double-Blind, Comparison of Tenofovir DF (TDF) versus Adefovir Dipivoxil (ADV)
for the Treatment of HBeAg Positive Chronic Hepatitis B (CHB): Study GS-US-174-0103
|
| |
| |
Reported by Jules Levin
AASLD, Nov 2-6, 2007, Boston, MA
J Heathcote1, E Gane2, R DeMan3, S Lee4, R Flisiak5, M Manns6, K Tchernev7, O Kurdas8, M Shiffman9, J Sorbel10, J Anderson10, E Mondou10, F Rousseau10
1University of Toronto, Toronto Canada; 2Middlemore Hospital, Auckland New Zealand; 3Erasmus MC, University Medical Center Rotterdam, The Netherlands; 4University of Calgary, Calgary AB Canada; 5Medical University of Bialystok, Bialystok Poland;
6Medizinische Hochschule, Hannover, Germany; 7Medical University, Sofi a, Bulgaria; 8Haydarpapa Numune Hospital, Istanbul Turkey; 9Virginia Commonwealth University, Richmond VA, USA; 10Gilead Sciences, Durham NC, USA
AUTHOR CONCLUSIONS
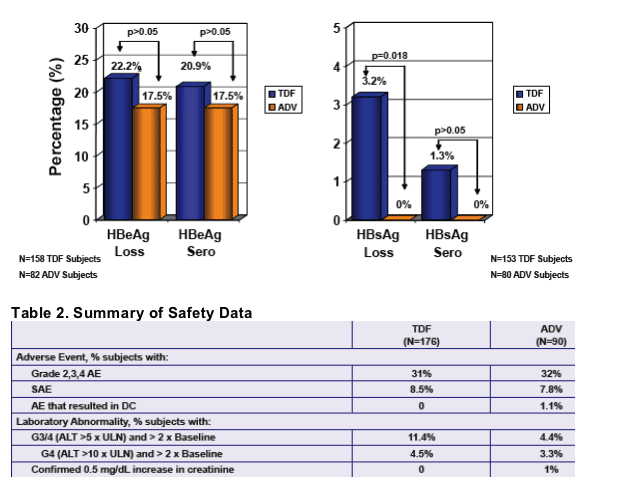
Tenofovir demonstrated superior efficacy to adefovir:
- 76% HBV DNA < 400 copies/mL
- 69% Normal ALT
- 3.2% HBsAg Loss
No subject developed mutations associated with tenofovir resistance
Tenofovir was well tolerated
Introduction
Tenofovir DF (TDF) is a nucleotide analogue
- Inhibits HIV-1 RNA transcriptase and HBV DNA polymerase
- Obligate chain terminator
TDF shown to have activity in patients mono-infected with HBV and co-infected with HIV/HBV
TDF has 6 years clinical safety data and 1.3 million patient years safety exposure in HIV
Aim of Study:
To evaluate the efficacy and safety of TDF versus adefovir dipivoxil (ADV) in
subjects mono-infected with HBeAg Positive CHB
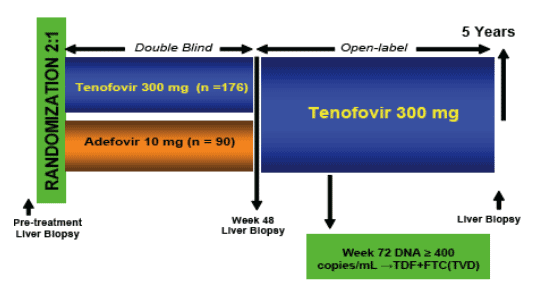
Figure 1. Study Design. 175 patients randomized to tenofovir and 90 to adefovir for 48 weeks with pre-treatment and 48 week liver biopsies performed. This is followed with 4 years of tenofovir open-label for a total 5 year study with a liver biopsy at year 5 performed. At week 72 FTC will be added if HBV DNA IS >400 C/ML.
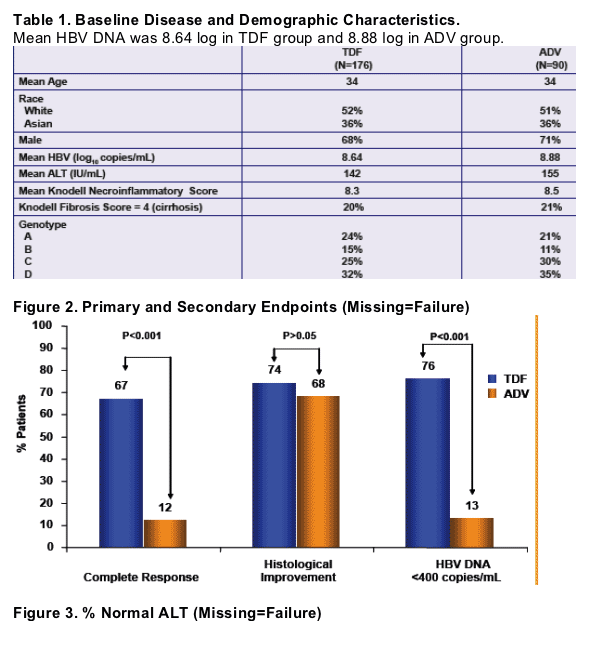
Study Endpoints (Week 48)
Primary Endpoint:
Complete Response: HBV DNA <400 copies/mL and histologic improvement (at least a 2-point reduction in the Knodell necroinfl ammatory score without worsening in fi brosis)
Secondary Endpoints:
-HBV DNA < 400 copies/mL
-Normal ALT
-HBeAg and HBsAg loss and seroconversion
-Resistance surveillance
-Histologic Improvement
METHODS
Key eligibility criteria:
-treatment naive, HBeAg+,18-69 years of age with compensated liver disease
-HBV DNA >106 copies/mL and ALT ≥ 2x and < 10xULN
-Knodell Necroinfl ammatory score ≥ 3; HIV, HDV, HCV negative
HBV DNA, serology, serum chemistry, liver tests, hematology, and urinalysis were evaluated every 4-12 weeks
HBV DNA measured using Roche COBAS TaqMan assay (LLOQ=169 copies/mL)
Resistance surveillance performed at Week 48 for all viremic subjects (≥ 400 copies/mL)
Treatment differences were evaluated using a z-test on the null hypothesis
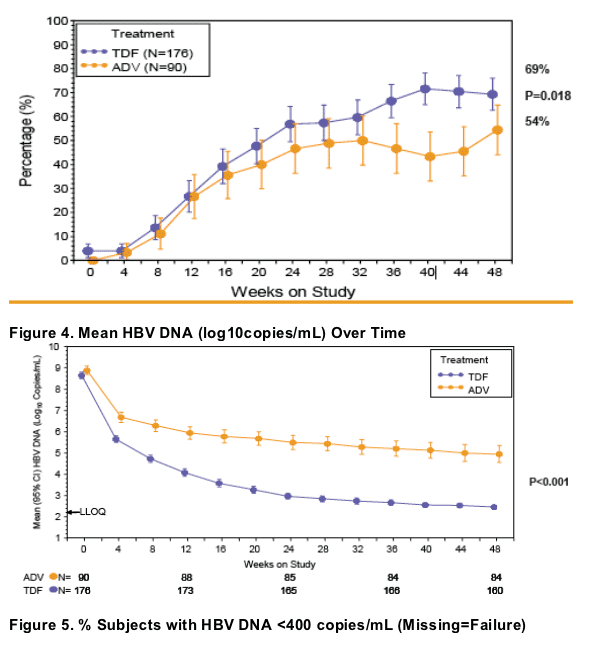
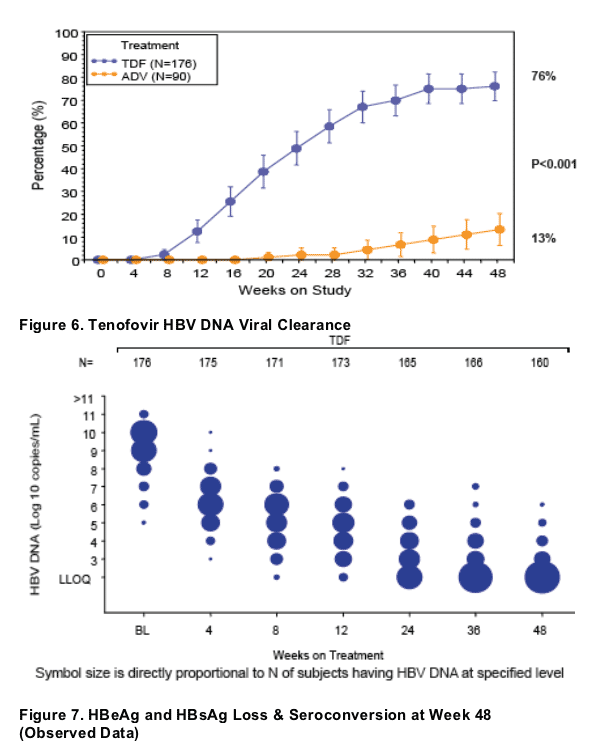
Resistance Surveillance
Evaluated all TDF-treated subjects with HBV DNA ≥ 400 copies/mL at Week 48 with or without viral breakthrough (defi ned as a confi rmed and consecutive 1 log increase in HBV DNA from nadir or HBV DNA ≥ 400 copies/mL after being <400 copies/mL)
No subject developed mutations associated with TDF resistance
|
| |
|
 |
 |
|
|