 |
 |
 |
| |
Efficacy of Tenofovir DF for the Treatment of Adefovir Resistance
|
| |
| |
Reported by Jules Levin
AASLD, Nov 2-6, 2007, Boston, MA
Poster 960.
van Bommel F1, Trojan J2, Wasmuth H3, Huppe D4, Moller B5, Feucht HH6, Wiedenmann B1, Berg T1
1Medizinische Klinik mit Schwerpunkt Hepatologie und Gastroenterologie, Charite Universitatsmedizin, Berlin; 2Department of Internal Medicine, Division of Gastroenterology, Johann Wolfgang Goethe University Medical Centre, Frankfurt, Germany; 3Medizinische Klinik III, Universitatsklinikum Aachen, Germany; 4Gastroenterologische Schwerpunktpraxis, Herne; 5Hepatologische Schwerpunktpraxis, Berlin; 6Institut fur Infektionsmedizin Universitatsklinikum Hamburg-Eppendorf, Germany.
Author Conclusions
In contrast to our initial impression, with longer follow up, TDF monotherapy shows significant activity against different patterns of ADV-resistant HBV mutations. In particular, no patient developed a viral breakthrough. More data and longer-term follow up are warranted to fully assess the efficacy of TDF in patients harbouring adefovir resistant HBV.
BACKGROUND
Genotypic HBV resistance against adefovir dipivoxil (ADV) develops rarely during the first two years of treatment. However, with increasing treatment duration, ADV-resistant HBV mutants are selected in up to 28% of the patients after 5 years, which is often associated with viral rebound. The mutations which mediate ADV resistance were described to be rtN236T and rtA181T/V (Fig 1). In vitro, the mutation rtN236T was described to cause a 3-14-fold decrease and the mutation rtA181T/V to cause a 1-3-fold decrease of ADV efficacy. Tenofovir disoproxil fumarate (TDF), a nucleotide analogue approved for HIV therapy, is closely related to ADV, shows equipotent antiviral activity against HBV on molar basis in vitro, and has some cross-resistance to ADV. Accordingly, in vitro analyses have demonstrated a 5-fold and a 1-fold decrease in TDF susceptibility for the rtN236T and the rtA181V mutations, respectively. However, due to the higher dosage of TDF (300 mg/d) as compared to ADV (10 mg/d), TDF might still be effective in ADV-resistant HBV infections.
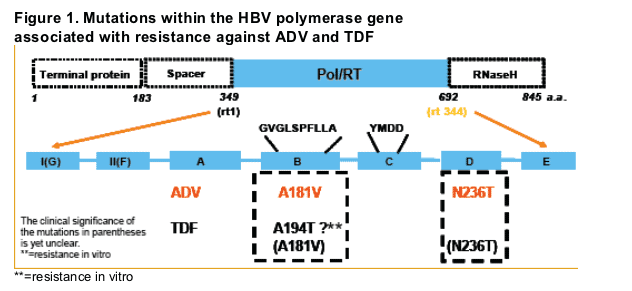
Aim of the study
The documentation of the antiviral efficacy of TDF in HBV monoinfected patients with genotypic resistance against ADV
Methods
In a multicenter retrospective study, 153 patients were identified who were treated with TDF 300 mg/d monotherapy for HBV monoinfections after preceding
failure of treatment with other nucleos(t)ide analogues (see abstract 83, AASLD meeting 2007). Effect of TDF therapy was analyzed in a subset
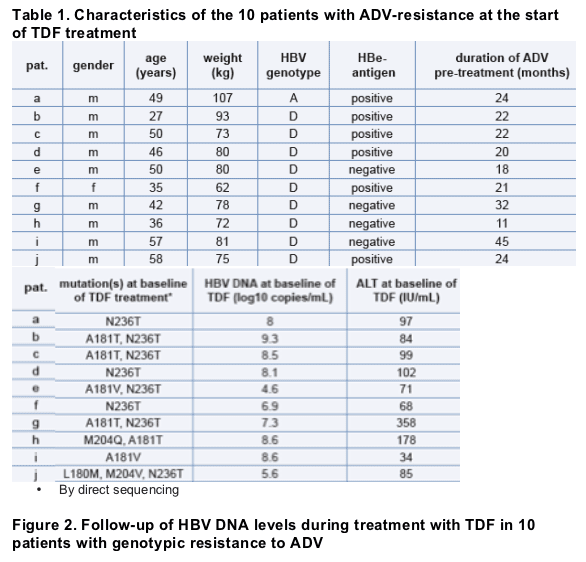
of 10 patients with genotypic ADV resistance and a duration of TDF treatment
≥ 12 months. The preceding treatment duration with ADV 10 mg/d was
11-45 (mean 24±9) months and all patients had history of lamivudine resistance
(see table 1 for baseline characteristics). During TDF treatment, ALT and HBV DNA values were measured every 3-6 months (COBAS TaqMan, Roche, Germany, detection limit 35 (1.54 log10) copies/mL). The HBV polymerase gene was sequenced from codon rt88 to rt282 after PCR products were cloned in E. coli (TOPO-TA cloning system, Invitrogen) from available frozen serum samples representing time points at the start and during TDF therapy.
RESULTS
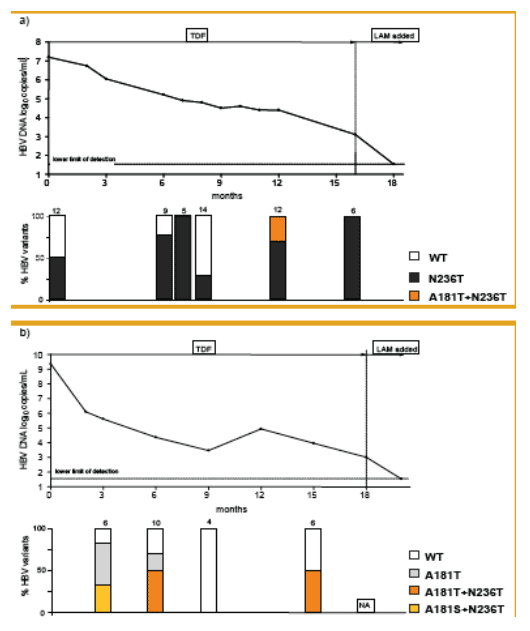
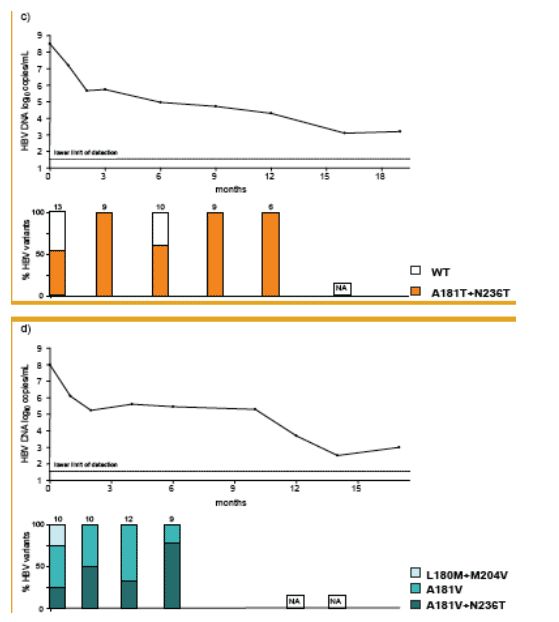
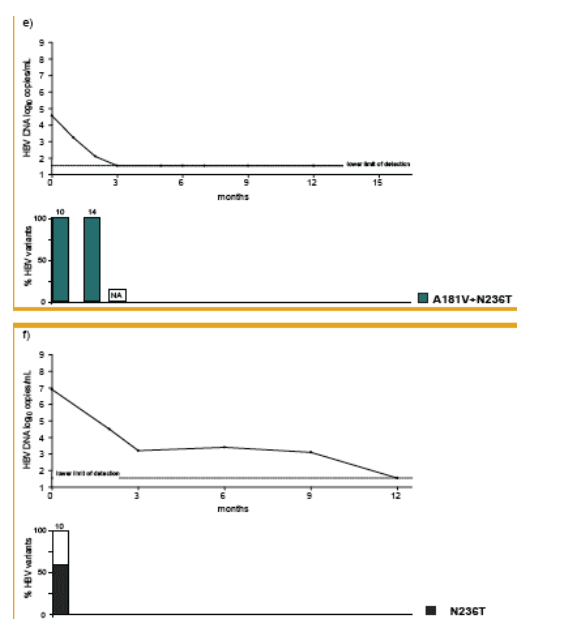
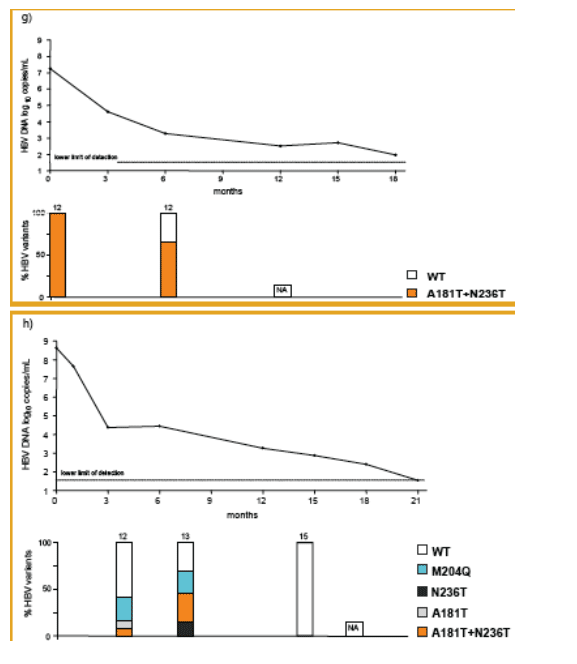
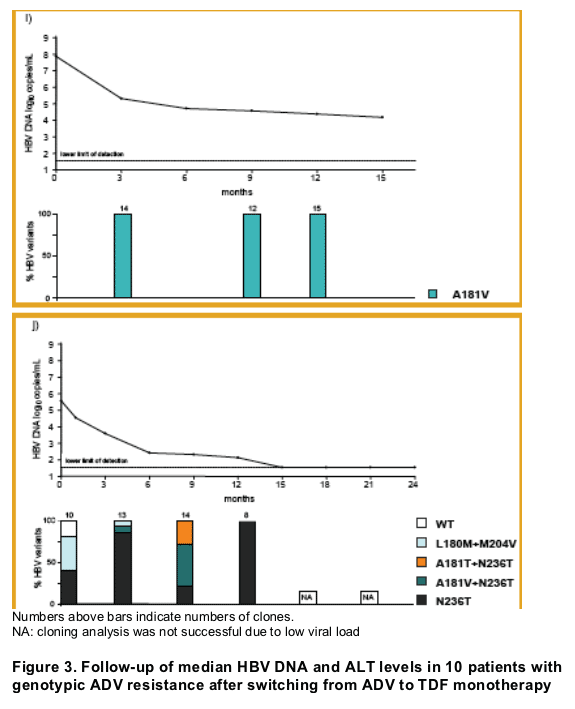
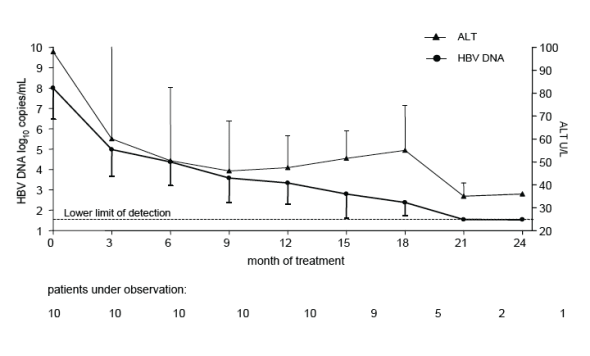
At the beginning of TDF treatment, the median HBV DNA was 7.6 [4.6-9.4] log10 copies/mL and nine patients had elevated ALT levels (median 98 [34-358] U/mL). After ADV was switched to TDF monotherapy, HBV DNA decreased by a median of 3.3 [2-5] and 4.4 [2.8-5.5] log10 copies/mL at months 6 and 12, respectively. HBV DNA was still detectable in 8 out of the 10 patients at month 12 of TDF treatment (median HBV DNA 3.3 [1.54-4.9] log10 copies/mL; assay LOD 35 [1.54 log10] copies/mL). At the end of the observation (mean duration 17±2.4 [12-24] months), 6 patients had HBV DNA < 1,000 copies/mL and 5 patients had HBV DNA <400 copies/mL; the median HBV DNA was 2.5 [1.54-4.9] log10 copies/mL. Subspecies analysis showed that ADV resistance mutations remained detectable throughout the observation period, in some patients in increasing ratio as compared to HBV wild type, as wild type was probably suppressed to a greater degree by TDF. Combination therapy with TDF and lamivudine was started in two patients and led to a complete suppression of HBV DNA to undetectable levels in both after two months of combination treatment, as displayed in figures 2a and 2b.
|
| |
|
 |
 |
|
|