 |
 |
 |
| |
First Multicenter Evaluation of the Efficacy of Tenofovir in Nucleo(t)ide Analog Experienced Patients with HBV Monoinfection
|
| |
| |
Reported by Jules Levin
AASLD, Nov 2-6,2007, Boston, MA
Poster 83.
F. van Bommel1; R. A. De Man2; A. Erhardt3; D. Huppe4; K. Stein5; P. Buggisch6; W. Bocher7; C. Sarrazin8, 9; J. Trojan9; U. Spengler10; J. G. Reijnders2; B. Moller11; H. E. Wasmuth12; P. Rohde13; H. Feucht14; B. Wiedenmann1; T. Berg1
1. Medizinische Klinik m. S. Hepatologie und Gastroenterologie, Charite-Universitatsmedizin Berlin, Berlin, Germany.
2. Department of Gastroenterology and Hepatology, Erasmus MC , University Medical Center Rotterdam, Rotterdam, Netherlands.
3. Klinik fur Gastroenterologie, Hepatologie und Infektiologie, Universitatsklinikum der Heinrich-Heine-Universitat Dusseldorf, Dusseldorf, Germany.
4. Gastroenterologische Gemeinschaftspraxis , Herne, Germany.
5. Innere Medizin IV, Universitatsklinikum Heidelberg, Heidelberg, Germany.
6. I. Medizinische Klinik, Universitatsklinikum Hamburg-Eppendorf, Hamburg, Germany.
7. I. Medizinische Klinik und Poliklinik, Johannes Gutenberg Universitat Mainz, Mainz, Germany.
8. Klinik fur Innere Medizin II, Universitatsklinikum des Saarlandes, Homburg an der Saar, Germany.
9. Medizinische Klinik I, Johan Wolfgang Goethe Universitat, Frankfurt am Main, Germany.
10. Zentrum fur Innere Medizin, Universitatsklinikum Bonn, Oklahoma, OK, USA.
11. Hepatologische Schwerpunktpraxis, Berlin, Germany.
12. Medizinische Klinik III, Universitatsklinikum Aachen, Aachen, Germany.
13. Abteilung fur Gastroenterologie, St. Marien Hospital, Hamm, Germany.
14. Institut fur Medizinische Mikrobiologie und Immunologie, Universitats-Krankenhaus Eppendorf, Hamburg, Germany.
Funded in part by Gilead Sciences
AIMS OF THE STUDY
Evaluation of long-term efficacy and safety of tenofovir fumarate (TDF) monotherapy in treat-ment experienced patients with HBV monoinfection.
Frequency of viral breakthrough due to HBV resistance.
Author summary
Treatment with TDF in this cohort of treatment-experienced patients resulted in potent suppression of HBV DNA.
Response to TDF was independent of preceding therapy with either lamivudine or adefovir, including presence of lamivudine resistance mutations.
Virologic breakthrough was not observed during the follow-up period.
Favorable safety and tolerability profile was observed with TDF.
Author conclusion
TDF is an effective and well tolerated treatment option for HBV monoinfected patients with prior treatment experience.
STUDY DESIGN
Retrospective cohort analysis of the follow up of treatment with TDF 300 mg qd monoinfection in HBV-monoinfected patients.
15 centers participated in Germany and The Netherlands.
A limitation of retrospective studies is the potential for selection bias:
--Therefore, data from all patients with HBV monoinfection and TDF treatment were collected.
Frozen serum samples from baseline were obtained if available.
ENDPOINTS
Primary endpoint
HBV DNA below LLOQ (<400 C/ML, Cobas Amplicor ASSAY, Roche)
Secondary endpoints
HBeAg and HBsAg loss/seroconversion
Normal ALT
Resistance development
Safety and tolerability
METHODS
Inclusion criteria
--chronic HBV monoinfection
--baseline HBV DNA > 10-4th c/ml at start of TDF treatment
--treatment with TDF >6 months
all patients had given informed consent for treatment with TDF.
Clinical and lab data (HBV DNA levels, ALT, creatinine) collected prospectively were retrospectively analyzed from TDF baseline and every 3-6 months during TDF treatment.
Screening for HBV resistance mutations at baseline TDF from frozen serum samples (direct sequencing).
RESULTS
153 patients with chronic HBV monoinfection were treated with TDF at the participating centers between 2002 and 2006.
45 patients were excluded due to:
--HBV DNA <10-4TH c/ml at TDF baseline (n=6)
--treatment with TDF < 6 months
--non-compliance to TDF as reported by treating physician (n=7)
--genotypic resistance to adefovir at baseline TDF (n=19); abstract 960.
108 patients in analysis population, median time on TDF treatment 20 [6-54] months.
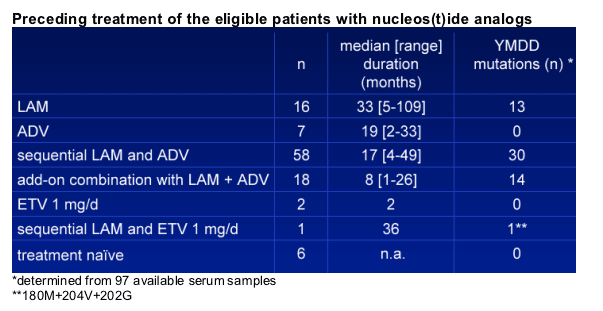
-93/108 (86%) patients were lamivudine experienced
-83/108 (77%) patients were adefovir experienced
-58/97 (60%) had proof of YMDD mutations
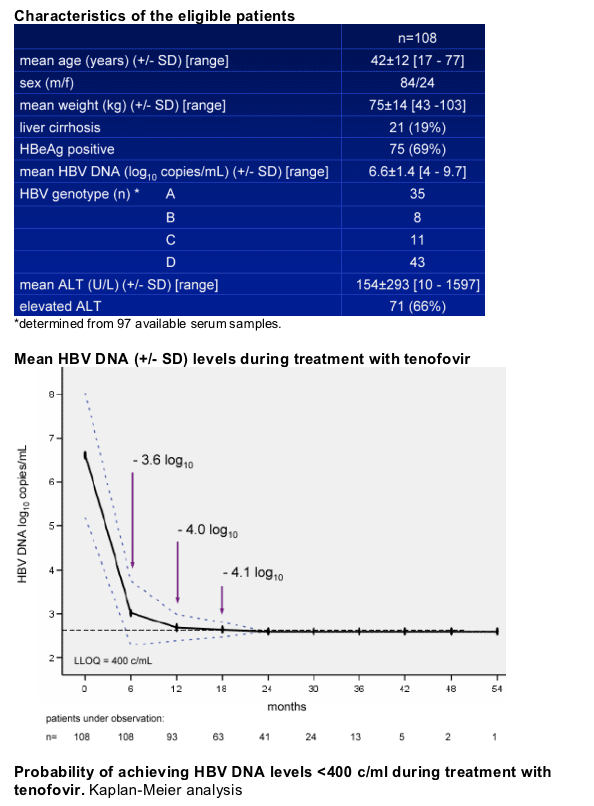
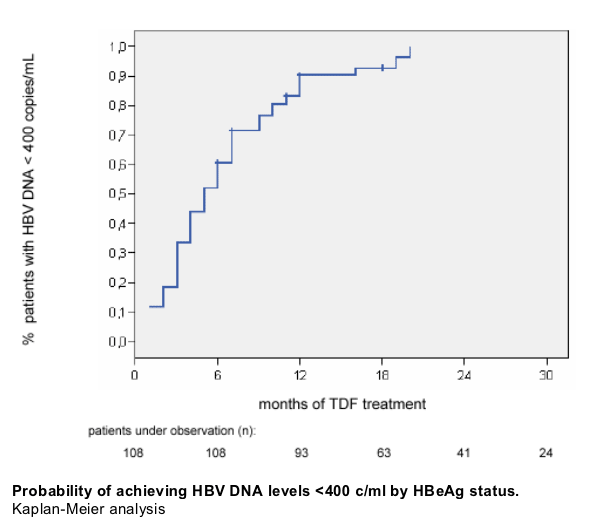
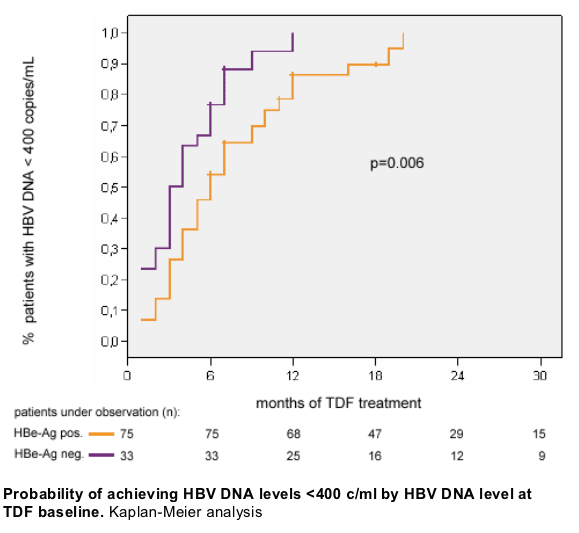
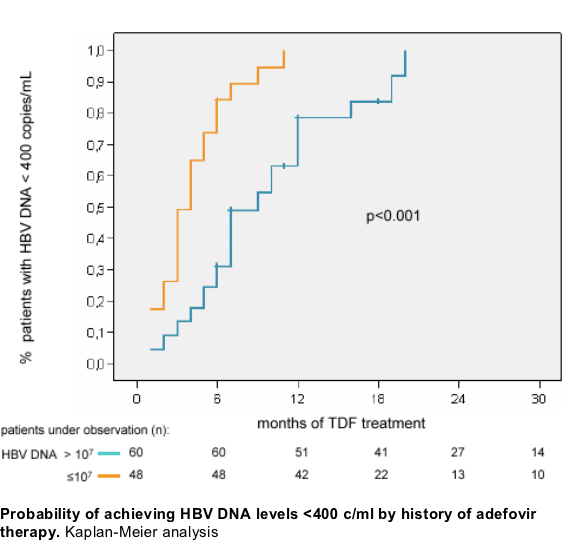
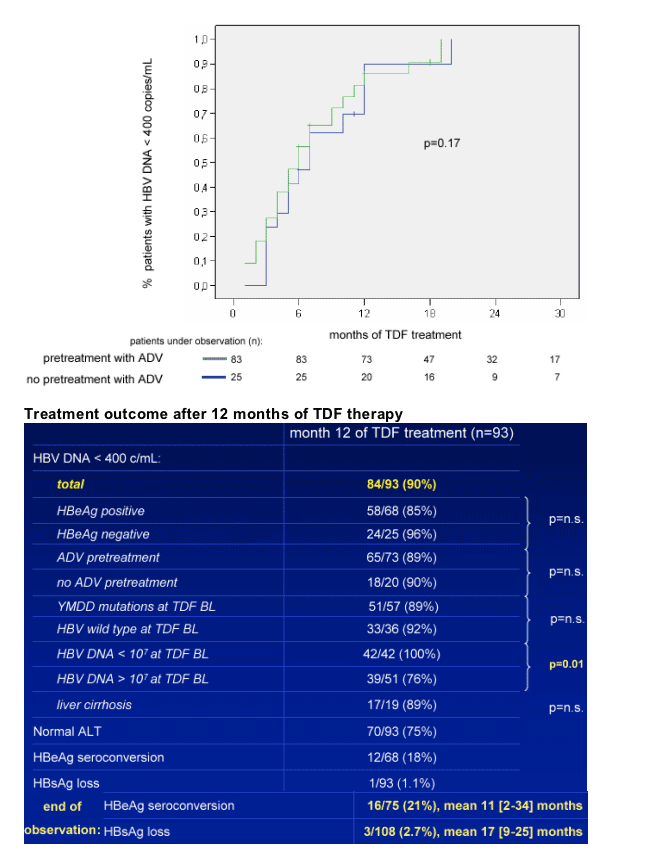
Safety and tolerability
During the followup period (median 20 months), no clinically significant side effects related to TDF were reported.
One patient showed mild creatinine elevation at month 12; creatinine levels remained within normal range in all other patients.

No virologic breakthrough (increase of HBV DNA >1 log c/ml from nadir) observed.
|
| |
|
 |
 |
|
|