 |
 |
 |
| |
Long-term follow-up of HBsAg clearance in patients with HBeAg-negative CHB treated with peginterferon alfa-2a (PEGASYS):
increase in HBsAg clearance rate from 3% 6 months post-treatment to 8% after 3 years
|
| |
| |
Reported by Jules Levin
AASLD, Nov 2-6, 2007, Boston, MA
P. Marcellin1, M. Brunetto2, F. Bonino3, G.K.K. Lau4, P. Farci5, C. Yurdaydin6, T. Piratvisuth7, K. Luo8, Y. Wang9, S. Hadziyannis10, E. Wolf11, M. Popescu12
1H˘pital Beaujon, University of Paris, Clichy, France. 2UO Gastreonterologia ed Epatologia, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy. 3Scientific Direction, Foundation IRCCS Policlinico of Milan and University of Pisa, Italy. 4Department of Medicine, Queen Mary Hospital, Hong Kong, China.
5Dipartimento di Scienze Mediche, UniversitÓ di Cagliari, Monserrato, Italy. 6Department of Gastroenterology, University of Ankara, Turkey. 7Department of Internal Medicine, Songklanagarind Hospital, Prince of Songkla University, Hat Yai, Thailand.
8Department of Infectious Diseases, Nanfang Hospital, Guangzhou, China. 9Infectious Disease Department, Xinan Hospital, Chongqing, China. 10Department of Medicine and Hepatology, Henry Dunant Hospital, Athens, Greece. 11MUC Research GmbH, Munich, Germany. 12Roche, Basel, Switzerland.
INTRODUCTION
HBsAg seroconversion (loss of serum HBsAg and development of anti-HBs antibodies) is the hallmark of successful immunological control of chronic hepatitis B (CHB) virus infection and is associated with lower incidence of cirrhosis and hepatocellular cancer (HCC) and improved survival rates1-3
HBsAg clearance/anti-HBs seroconversion can be induced by interferon therapy,
with rates of HBsAg clearance of up to 30% reported in patients with HBeAg-negative CHB who were followed up long-term after having a sustained response to interferon treatment4
The ability of interferon-based therapy to induce HBsAg loss/seroconversion may be linked to the dual immunomodulatory and antiviral properties of interferon since this is rarely achieved in patients treated with direct antiviral therapies, i.e. nucleot(s)ide analogs, alone5
HBsAg clearance can also be achieved through treatment with peginterferon alfa as demonstrated recently in randomized trials of peginterferon alfa-2a and -2b in patients with HBeAg-positive and HBeAg-negative and HBeAg-positive chronic hepatitis B6-8
HBsAg clearance during the long-term follow-up of patients with HBeAg-negative
chronic hepatitis B treated peginterferon alfa-2a has not been documented
Summary
The proportion of PEGASYS-treated patients with HBsAg loss increased over time from 3-4% 6 months post-treatment to 8% 3 years post-treatment
- In contrast, HBsAg clearance was not observed in any patient treated with lamivudine alone
8 (44%) of the patients who cleared HBsAg had also developed anti-HBsAg 3 years posttreatment
HBsAg clearance was observed across all major genotypes with a higher frequency in patients infected with HBV genotype A
HBsAg level at week 48 was predictive of subsequent HBsAg level 3 years post-treatment
While 17/18 patients who cleared HBsAg had levels of HBV DNA suppressed to <400 copies/mL at end of treatment and during the follow-up period, HBV DNA suppression to <400 copies/mL at the end of treatment was not predictive of HBsAg clearance 3 years post-treatment with PEGASYS
- Thus, HBV DNA suppression appears to be required, but is not sufficient, for
subsequent clearance of HBsAg
- The propensity of interferon-based therapy to induce HBsAg clearance may be
associated with its dual immunomodulatory and antiviral mode of action
CONCLUSION
PEGASYS-induced HBsAg clearance (after 48 weeks of treatment) occurred during long-term follow-up of patients with HBeAg-negative disease
Quantification of on-treatment reduction in HBsAg level may be useful for predicting response to PEGASYS treatment
The ability to induce HBsAg clearance, an outcome that is associated with long-term improvement in survival, supports the use of PEGASYS as a first-line treatment for patients with HBeAg-negative CHB
OBJECTIVE
To determine HBsAg clearance up to 3 years post-treatment in patients from a study of peginterferon alfa-2a [40KD] (PEGASYS) ± lamivudine versus lamivudine alone in patients with HBeAg-negative CHB who entered a long-term roll-over study
To quantify HBsAg level prior to treatment and at the end of treatment and to identify potential factors that could be useful for predicting subsequent HBsAg clearance 3 years post-treatment
METHODS
Initial study
537 patients were randomized 1:1:1 to receive 48 weeks of treatment with:
- 180 _g/week PEGASYS plus oral placebo daily (n=177)
- PEGASYS 180 _g/week plus 100 mg/day lamivudine (n=179)
- 100 mg/day lamivudine (n=181)
All patients were positive for HBsAg and negative for anti-HBs antibody pre-treatment
Loss of HBsAg and presence of anti-HBs antibody was assessed at end of treatment (week 48) and 6 months post-treatment (week 72)
Quantitative HBsAg (qHBsAg) was measured retrospectively pre-treatment, at the end of treatment (week 48) and 6 months post-treatment (week 72) for a subset of patients:
-- n=127 from the PEGASYS arm; n=137 from the PEGASYS plus lamivudine arm; n=122 from the lamivudine arm
Long-term follow-up study
All patients who were randomized, received treatment and returned for follow up in the initial study were eligible to enter the 3-year observational follow-up protocol (long-term follow-up study)
315 of 386 eligible patients entered the long-term observational follow-up protocol:
- n=116 had received PEGASYS plus oral placebo
- n=114 had received PEGASYS plus lamivudine
- n=85 had received lamivudine only
Patients receiving either registered or investigational therapy for CHB during the
follow-up period were excluded from the follow-up phase and were regarded as
nonresponders in the observational protocol
Patients who had an alanine aminotransferase (ALT) value >2 x ULN or HBV DNA levels >100,000 copies/mL at 48 weeks post-treatment discontinued the study and were considered to be non-responders in the analyses
Loss of HBsAg and presence of anti-HBs antibody was assessed at 1, 2 and 3 years after the end of treatment
Quantitative HBsAg determination (qHBsAg)
HBsAg was quantified using the Architect HBsAg assay (Abbott Laboratories; dynamic range 0.05-250.0 IU/mL) after 1:100 dilution. Samples with HBsAg levels higher than 250.0 IU/mL at 1:100 dilution were retested at a final dilution of 1:1,000. Samples with HBsAg levels <0.05 IU/mL at 1:100 dilution were retested undiluted
Statistical analysis
Logistic regression analysis was used to identify potential factors that could be associated with HBsAg loss 3 years post-treatment
Factors investigated included: age, baseline ALT, baseline HBV DNA, HBsAg level at week 48, and treatment with PEGASYS vs PEGASYS + LAM
RESULTS
Patient disposition
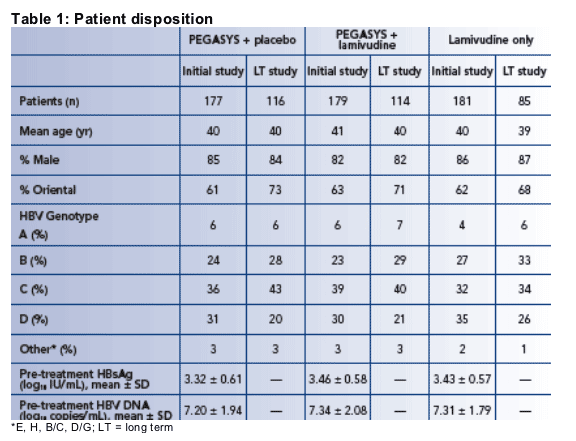
Baseline characteristics, genotypic distributions and HBsAg and HBV DNA levels of patients in the initial and long-term studies, and in the individual study arms, were very similar (Table 1)
The mean pretreatment HBsAg level was 3.4 log10 (2,500) IU/mL; 21.5% of patients had HBsAg < 3 log10 (1,000) IU/mL, 63% of patients had HBsAg ≥ 3 but ≦ 4 log10 (1,000-10,000) IU/mL, 15.3% of patients had HBsAg ≥ 4 log10 (10,000) IU/mL
HBsAg clearance and seroconversion rates during long-term follow-up (up to 3 years post-treatment)
The HBsAg clearance rates 6 months post-treatment were 3%, 4% and 0% in patients treated with PEGASYS, PEGASYS plus lamivudine and lamivudine alone
The number and percentage of patients who cleared HBsAg during years 1, 2 and 3 post-treatment is shown in Table 2
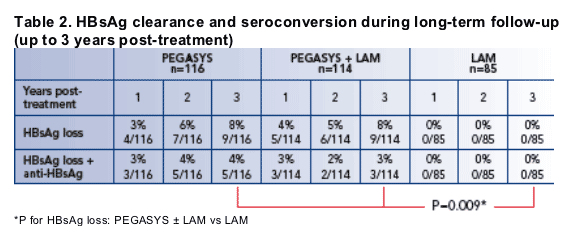
In total, 18 patients (8%) treated with PEGASYS monotherapy or PEGASYS in combination with lamivudine who entered the long-term follow-up study had cleared HBsAg at 3 years post-treatment
6 of the 18 patients were HBsAg-positive at the 2 year post-treatment follow-up and so cleared HBsAg during the 3rd post-treatment follow-up year
8 (44%) of the 18 PEGASYS-treated patients who had cleared HBsAg at 3 years had developed anti-HBs antibodies
No patient in the lamivudine only arm had cleared HBsAg at 3 years post-treatment
Profile of patients who cleared HBsAg 3 years post-treatment
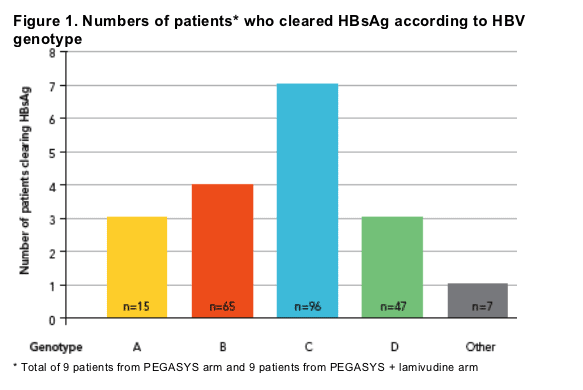
Clearance of HBsAg across all common genotypes
Loss of HBsAg was observed across all the major HBV genotypes (Figure 1)
HBV DNA profile of PEGASYS-treated patients who cleared HBsAg 3 years post-treatment
All but 1 patient who cleared HBsAg had HBV DNA <400 copies/mL during the 3-year follow-up period
16 of 17 patients for whom data were available had HBV DNA levels <400 copies/mL at the 3-years post-treatment evaluation
- The remaining patient (who received PEGASYS plus lamivudine) had an HBV DNA level of 1,331 copies/mL
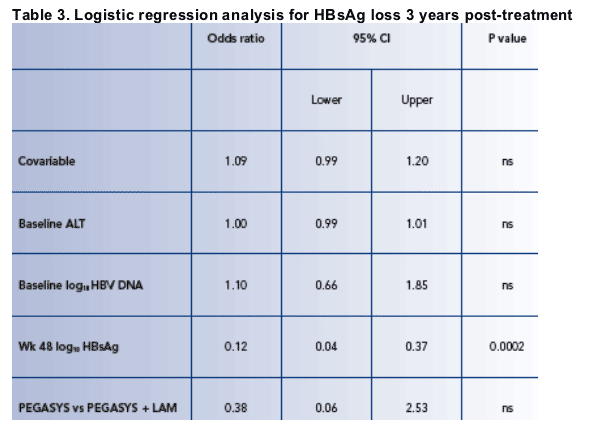
Prediction of response 3 years post-treatment
- End of treatment HBsAg level as a predictor of subsequent HBsAg clearance 3 years post-treatment
As no HBsAg clearance was seen in patients entering the long-term study from the lamivudine only arm, the results described in this section concern only patients who received peginterferon alfa-2a (± LAM)
Long-term data on HBsAg loss 3 years post-treatment were available for 65 patients for whom qHBsAg was available
- 34 treated with PEGASYS
- 31 treated with PEGASYS + LAM
Log10 HBsAg level at week 48 was a significant predictor of HBsAg loss 3 years posttreatment by logistic regression analysis (Table 3) with an odds-ratio of 0.12 (95% CI 0.04, 0.37; p=0.0002)
- For each unit decrease in log10 HBsAg at week 48 the odds of HBsAg loss at year 3 increase by a factor of 0.88, ie an 88% increase in the chance of HBsAg loss at year 3
Age, baseline ALT, baseline HBV DNA and treatment with PEGASYS vs PEGASYS + LAM were not predictive of HBsAg loss 3 year post-treatment (Table 3)
End of treatment (week 48) HBV DNA level and subsequent HBsAg clearance 3 years post-treatment
Although all except one patient who had cleared HBsAg by 3 years had HBV DNA suppressed to <400 copies/mL at week 48, HBV DNA <400 copies/mL was not predictive of HBsAg clearance
Thus, HBV DNA suppression is required for, but is not sufficient for, HBsAg clearance
REFERENCES
1. Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology 2004;39:857-61
2. de Frandchis R et al. EASL International Consensus Conference on Hepatitis B. 13 - 14 September, 2002; Geneva, Switzerland: consensus statement (long version). J Hepatol 2003;39(Suppl 1):S3-S25
3. Fattovich G et al. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alpha therapy and disease prognosis. Am J Gastroenterol 1998;93:896-900
4. Lampertico P et al. Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology 2003;37(4):756-63
5. Hadziyannis SJ et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. New Engl J Med 2005;352(26):2673-81
6. Marcellin P et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with for HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206-17
7. Lau GK et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352(26):2682-95
8. Janssen HLA et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005;365(9454):123-29
DISCLOSURE INFORMATION
This research was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland
|
| |
|
 |
 |
|
|