 |
 |
 |
| |
Reduction in serum HBsAg level in patients with chronic hepatitis B infected with genotype D induced by (pegylated) interferon alfa-2a alone or in combination with nucleos(t)ide analogs: a long-term single centre cohort study
|
| |
| |
Reported by Jules Levin
AASLD, Nov 2-6, 2007, Boston, MA
Brunetto MR, Moriconi F, Cavallone D, Oliveri F, Maina AM, Ciccorossi P, Colombatto P, Coco B, Moscato G* and Bonino F**
UO Gastreonterologia ed Epatologia, *Laboratorio Centrale,Azienda Ospedaliera Universitaria Pisana, Pisa, Italy and ** Scientific Direction, Foundation IRCCS Policlinico of Milan and University of Pisa, Italy
INTRODUCTION
In patients with chronic hepatitis B (CHB) undergoing antiviral therapy, clearance of serum HBsAg appears to be associated with favorable long-term clinical outcome.1-5
Patients treated with interferon-based therapies achieve higher rates of HBsAg clearance than those treated with nucleos(t)ide analogs (NAs).6-8 A recent study suggested that on-treatment HBsAg quantification may provide clues about the likelihood of subsequent HBsAg clearance.9
In clinical practice, quantification of serum HBsAg might provide a valuable tool to identify the best therapeutic strategy for individual patients.
STUDY AIM
To quantify serum HBsAg in patients with HBeAg-negative CHB who were treated at our chronic viral hepatitis reference center with interferon (IFN)/peginterferon alfa-2a (PEGASYS), with or without nucleos(t)ide analogs (NAs), or NAs alone
Summary
Over a mean follow-up of 40 months HBsAg was cleared by 11% of patients treated with IFN/PEGASYS-based therapy
In contrast no patient treated with NAs alone achieved HBsAg clearance and reductions in HBsAg level in these patients were minimal
One patient treated with PEGASYS following prior treatment failure with LAM cleared HBsAg
CONCLUSION
IFN/PEGASYS-based therapy appears to be most effective in reducing levels of serum HBsAg independent of baseline host and virologic features, including resistance to LAM
PEGASYS therapy could prove a successful rescue therapy for patients who have failed prior LAM therapy
Yearly quantification of serum HBsAg could provide a useful tool to predict the long-term outcome of IFN-based therapy and its ability to induce HBsAg clearance in CHB patients infected with genotype D
METHODS
We enrolled 80 patients (median age 54 years, range 17-78 years; 62 males, 18 females) with chronic hepatitis B and infected with HBV genotype D
Treatment regimens were as follows (Figure 1):
- 11 patients received interferon [IFN] alfa or peginterferon alfa-2a (PEGASYS)
monotherapy for 48 weeks
- 24 patients received PEGASYS + lamivudine (LAM): 11 in combination for 48 weeks and the remaining 13 with sequential schedules
- 6 patients received LAM or adefovir (ADV) monotherapy for 24-60 months
- 39 patients received LAM + ADV for 36-72 months
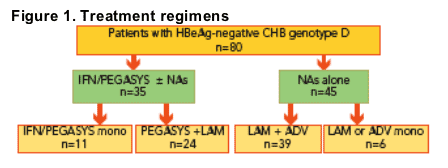
Of the 80 patients, 45 had resistance to LAM; 39 of whom received LAM + ADV and 6 of whom received PEGASYS + LAM
Treatment response was defined as:
- Normal ALT, HBV DNA level < 20,000 cp/mL and IgM anti HBc < 0.2 IMx index at every 3 monthly control for at least 12 months after the end of therapy for IFN/PEGASYStreated patients
- Persistently normal ALT with undetectable HBV DNA and IgM anti HBc <0.2 IMx index during long-term NA therapy
All patients were followed up for a median period of 3.5 years (range 2-7 years)
Assays
HBsAg was measured yearly over a median period of 3.5 years (range 2-7 years)
HBsAg was quantified using the Architect 2000 HBsAg assay (Abbott Laboratories; dynamic range 0.05-250.0 IU/mL). Samples with HBsAg levels higher than 250.0 IU/mL at 1:100 dilution were retested at a final dilution of 1:1000. Samples with HBsAg levels <0.05 IU/mL at 1:100 dilution were retested undiluted
HBV DNA was measured by Amplicor Roche (dynamic range 400-200,000 cp/mL), samples with viremia higher than 200,000 cp/mL were retested after 1:100 dilution according to manufacturers recommendations
Serum ALT was measured at time of sampling. Normal ALT was considered to be ≦1 x upper limit of normal (ULN; 30 IU/mL)
RESULTS
Baseline characteristics
Baseline characteristics such as gender, viral load and ALT level were not significantly different between the groups of patients (Table 1)
Patients treated with NAs had lower HBsAg baseline levels (mean 3.39 vs 3.77 log10 IU/mL, p=0.025) and were older (mean 57 vs 45 years, p<0.001) than patients in other groups
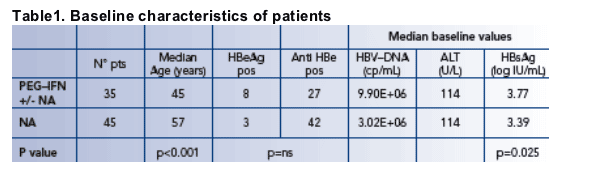
Response to therapy
The proportion of patients responding to therapy is shown in Figure 2
During treatment with NAs 42/45 patients (93.3%) achieved a complete inhibition of viral replication
- 36 of these were resistant to LAM and the control of viral replication was obtained with ADV rescue therapy
29/35 patients (82.9%) treated with IFN/PEGASYS had a response at the end of treatment
- 10/29 patients (34.5%) maintained the response throughout the post- treatment follow up
- 4 of these 10 patients (40%) cleared HBsAg
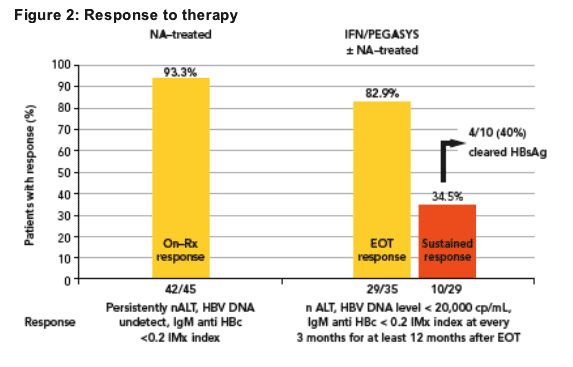
Change in HBsAg level
Reductions in HBsAg levels from baseline to last observation are shown in Figure 3 and Table 2
No patient treated with NAs alone cleared HBsAg
- Reductions in HBsAg levels in these patients were mostly small in magnitude
(<0.5 log10 IU/mL)
Half of the patients treated with IFN-based therapy had reduced HBsAg by ≥0.5 log10
Four patients (11%) who had been treated with IFN/PEGASYS-based therapy cleared HBsAg
- 1 of these had been received PEGASYS after having failed prior LAM treatment
Individual patient profiles showing HBsAg reduction, HBV DNA and ALT over time
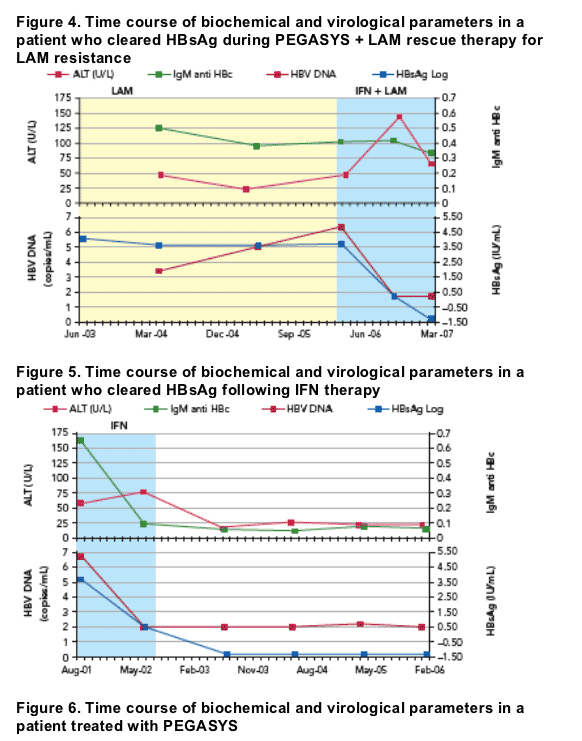
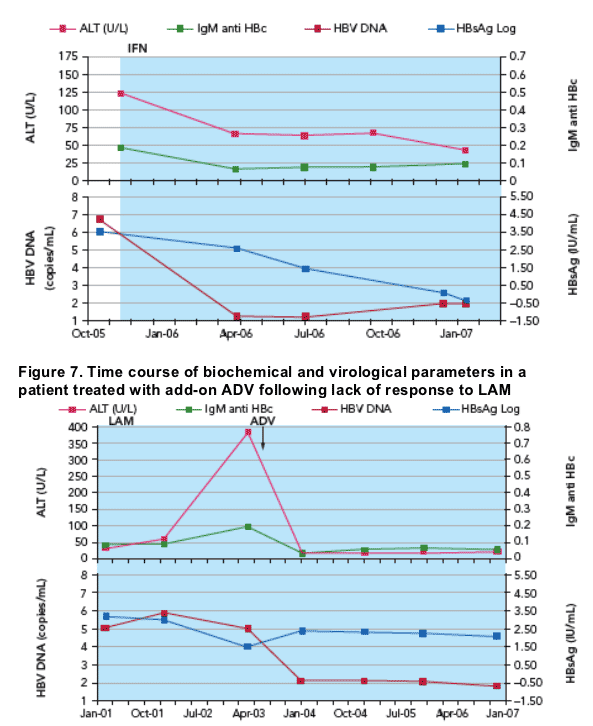
Individual profiles of patients are shown in Figures 4 to 7
- Figure 4 shows a patient who cleared HBsAg following rescue therapy with PEGASYS following failed LAM therapy
- A patient who cleared HBsAg following IFN therapy is shown in Figure 5
- Figure 6 illustrates the reduction in HBsAg level following treatment with PEGASYS
- In contrast, Figure 7 shows little effect on HBsAg levels of add-on ADV in a patient who was not responding to LAM
REFERENCES
1. Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology 2004;39:857-61
2. de Franchis R et al. EASL International Consensus Conference on Hepatitis B, 13-14 September, 2002, Geneva, Switzerland: consensus statement (long version). J Hepatol 2003;39(Suppl 1):S3-S25
3. Lok AS, McMahon BJ. AASLD practice guidelines 2003: chronic hepatitis B. www.aasld.org/netFORUMAASLD/eweb/docs/ chronichep_B.pdf.
4. Fattovich G et al. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alpha therapy and disease prognosis. Am J Gastroenterol 1998;93:896-900
5. Lin S-M et al. Interferon in HBeAg-positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol 2007;46:45-52
6. Brunetto M et al. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long-term cohort study. J Hepatol 2002;36:263-70
7. Lampertico P et al. Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology 2003;37:756-63
8. Marcellin P et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with for HBeAg-negative
chronic hepatitis B. N Engl J Med 2004;351:1206-17
9. Manesis EK et al. Quantitative analysis of hepatitis D RNA and hepatitis B surface antigen serum levels in chronic delta hepatitis improves treatment monitoring. Antivir Ther 2007;12:381-8
DISCLOSURE INFORMATION
This research was funded in part by F. Hoffmann-La Roche Ltd, Basel, Switzerland
|
| |
|
 |
 |
|
|