 |
 |
 |
| |
PK Atazanavir-based HAART in pregnancy
|
| |
| |
Reported by Jules Levin
CROI, Feb 2007, Los Angeles
D. Ripamonti1, D. Cattaneo2, M. Airoldi1, L. Frigerio1, P. Bertuletti1, M. Ruggeri1, G. Remuzzi 2,
F. Suter1, F. Maggiolo1
Ospedali Riuniti1, Mario Negri Institute for Pharmacological Research2, Bergamo, Italy
ABSTRACT
Background: Optimal antiretroviral exposure during pregnancy is critical to prevent MTCT of HIV. Pregnancy can alter antiretroviral pharmacokinetics. Our objective was to describe atazanavir/ritonavir (ATV/r) pharmacokinetic during pregnancy.
Methods: We performed intensive steady-state 24-hours pharmacokinetic profiles of ATV/r associated to AZT and 3TC (at standard doses) at 30-36 weeks gestation and 8-16 weeks postpartum. Maternal and umbilical cord blood samples were obtained at delivery. HPLC was used to measure ATV/r concentrations.
Results: 9 women completed ante/postpartum evaluations. Their mean age was 31 years and their mean baseline CD4 count was 471 cells/mcL. HAART was started between 0 and 24 weeks of pregnancy (mean 13.7).
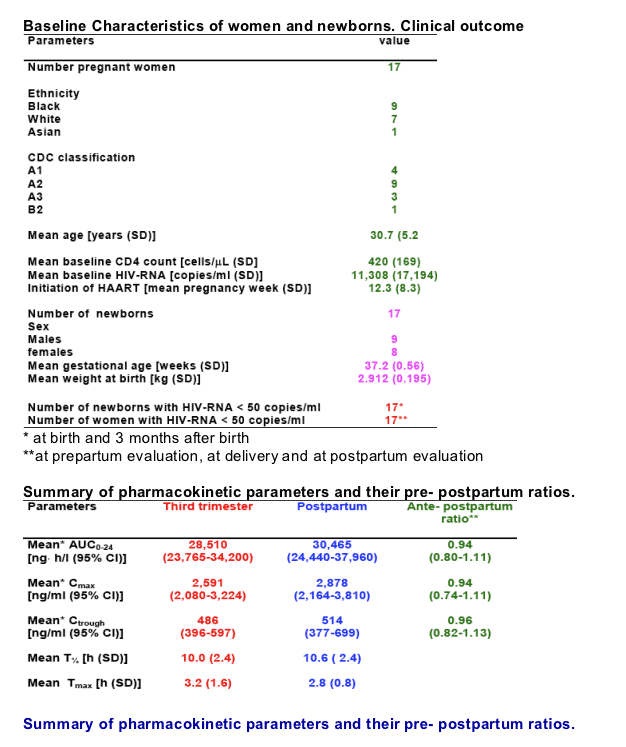
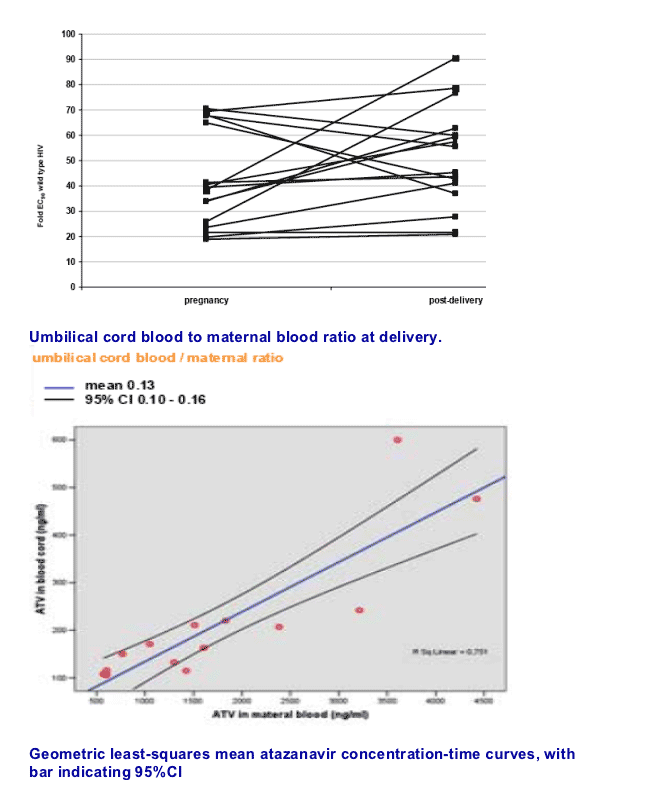
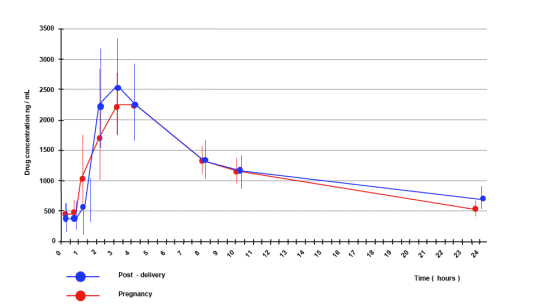
The mean antepartum ATV/r AUC was 29.4 mcg h/ml (SE 3.1), while the postpartum value was 33.5 mcg h/ml (SE 3.8) with a mean ante/postpartum ratio of 0.95 (SD 0.34). Antepartum ATV/r mean Cmax resulted of 2.7 mcg/ml (SE 0.4) compared to a postpartum value of 2.9 mcg/ml (SE 0.4) for a ante/postpartum ratio of 1.03 (SD 0.31). Tmax was of 3.1 h antepartum and 2.8 h postpartum, while Cmin resulted of 5.2 mcg/ml antepartum and 5.9 mcg/ml postpartum. None of the considered measures was significantly different (P > 0.05) nor they were influenced by the womenÕs BMI (mean 26.9 during and 24.5 after pregnancy). ATV/r mean concentration in the cord blood at delivery was 0.22 mcg/ml (SD 0.13) and counted for 9.7% of the concomitant maternal blood concentration 2.3 mcg/ml (SD 1.3). All newborns at delivery and 3 months after birth resulted HIV-RNA negative. None of them required phototherapy.
Conclusions: ATV/r concentrations during late pregnancy are similar to those obtained postpartum. No dose adjustments should be needed. Small amounts of ATV cross the placenta. An ATV/r based HAART is effective in preventing MTCT of HIV infection.
Data presented in this poster consider a population larger than that reported in the abstract. This larger casuistry leads to the same conclusions of the abstract and adds sufficient statistical power
POSTER CONCLUSIONS
Atazanavir overall exposure at steady state during the third trimester of pregnancy is similar to that observed in the non-pregnant state.
Over the whole dosing interval therapeutic drug concentrations are well above the wild-type HIV IC90 (approximately 40 folds).
Atazanavir crosses the placenta at a 13% ratio with clear positive linear correlation to maternal plasma levels. Consequently, even considering maternal Ctrough levels the fetus exposure to atazanavir would fell into a therapeutic range roughly 4 folds higher than wild-type HIV IC90. On the other hand, a limited, but still therapeutic, placental transfer of atazanavir may protect the fetus
against the potential toxic effects of the drug.
Since pregnancy does not appear to alter plasma exposure to atazanavir, no dose adjustment is required in pregnant women.
Pharmacokinetic results suggest that a standard boosted atazanavir dose is a
reasonable component of HAART during pregnancy.
Introduction
When compared with postpartum, lower AUC and Ctrough prepartum values have been reported for boosted LPV, boosted SQV, IDV and NFV. It is critical to understand the effect of pregnancy on absorption and disposition of different PIs, in order to ensure the best therapeutic choices and that adequate concentrations are achieved in the blood of pregnant women so to prevent the development of viral resistance and vertical transmission of the infection.
The primary purpose of this study was to evaluate if a standard boosted atazanavir dose produces adequate drug exposure during pregnancy. MTCT program. HIV-infected pregnant women receive HAART as soon as the HIV status is known (or continued in the case of a previously treated woman) if the
womanÕs immunological status requires immediate treatment, otherwise HAART is delayed until the end of the first trimester of pregnancy. Women undergo elective cesarean resection. At birth the children receive one month AZT prophylaxis (2 mg/kg every 6 hours). Breast feeding is avoided.
Definition of Equivalence
According to the Center for Drug Evaluation and Research. Draft Guidance for Industry: Pharmacokinetics in Pregnancy: Study Design, Data Analysis, and
Impact on Dosing and Labeling. Washington, DC: US Department of Health and Humans Services, Food and Drug Administration; October 2004.
Http://www.fda.gov/cder/guidance/5917dft.htm
A pharmacokinetic parameter was defined as equivalent if the 90% Confidence Interval (CI) of the prepartum to the postpartum ratio of observed values felt between 0.80 and 1.25. For geometric means the CI was calculated as the antilog of the true mean of the log ratios
|
| |
|
 |
 |
|
|