 |
 |
 |
| |
CCR5 Blocker Looks Strong in Two International Salvage Trials
|
| |
| |
Mark Mascolini
February 28, 2007
14th Conference on Retroviruses and Opportunistic Infections
Los Angeles
Plugging CCR5 receptors on CD4 cells, maraviroc reversed the virologic fortunes of most people with multidrug-resistant virus in two phase 3 trials detailed at the 14th Conference on Retroviruses [1,2]. Trial participants taking maraviroc did not suffer more new malignancies than people taking placebo, a concern that arose in an earlier study of the CCR5 antagonist vicriviroc [3]. But HIV in most people who endured virologic failure while taking maraviroc did swap coreceptor preference from CCR5 to CXCR4.
Howard Mayer and Elna van der Ryst from Pfizer spelled out results of MOTIVATE 1 and 2, which enrolled 1076 people with CCR5-tropic virus at screening and a viral load above 5000 copies/mL. Everyone had resistance to and/or experience with at least one drug from the first three antiretroviral classes, including two or more protease inhibitors. Researchers in Europe, Australia, and North America randomized enrollees to 150 mg of maraviroc once or twice daily or to placebo plus an optimized background regimen (OBR).
Median baseline CD4 counts stood at about 150 to 180 cells across the six arms of the two trials, and median starting viral loads measured about 4.8 log copies/mL. Approximately 40% of enrollees used enfuvirtide in their OBR. And about two thirds started two or fewer active drugs besides maraviroc.
After 24 weeks in MOTIVATE 1, the average viral load dropped 1.82 log in the once-daily maraviroc arm, 1.95 log in the twice-daily maraviroc arm, and 1.03 log in the placebo arm. Respective 24-week changes in MOTIVATE 2 were 1.95 log, 1.97 log, and 0.93 log. At the 24-week point of MOTIVATE 1, 60.4% in the once-daily arm, 54.7% in the twice daily arm, and 31.4% in the placebo arm had a viral load below 400 copies (P < 0.0001 for either maraviroc arm versus placebo). Sub-50-copy rates measured 48.5% with once-daily maraviroc, 42.2% with twice daily maraviroc, and 24.6% with placebo (P < 0.0001 versus placebo). Again, 24-week results in MOTIVATE 2 were similar. All of these analyses involved everyone who took at least one dose of maraviroc or placebo.
Average CD4 counts rose by 107 cells in the once-daily arm of MOTIVATE 1, by 111 cells in the twice-daily arm, and by 52 cells with placebo (P < 0.0001 versus placebo in a last-observation-carried forward analysis). CD4 changes proved similar in MOTIVATE 2.
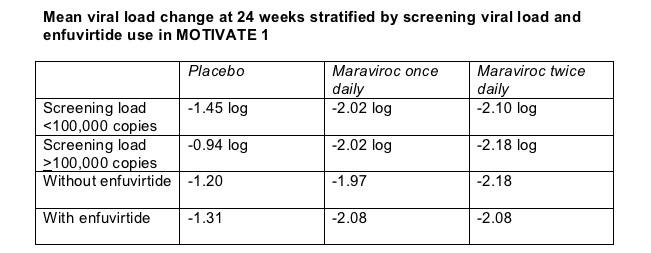
A starting viral load above 100,000 copies diminished 24-week virologic response in the placebo arm but not in the maraviroc arms of MOTIVATE 1 (Table). In the same way, adding the fusion inhibitor to the OBR in MOTIVATE 1 boosted 24-week virologic responses in the placebo arm, but people taking maraviroc gained little virologically by adding enfuvirtide (Table). Still, Howard Mayer told attendees he thinks enfuvirtide had an additive effect with maraviroc.
Earlier salvage trials demonstrate that having more active drugs in the background regimen helps a novel antiretroviral quell viral replication. The same proved true in MOTIVATE 1 and 2. Combined analysis of the two trials showed that 29% in the twice-daily maraviroc arm, 18% in the once-daily arm, and 3% in the placebo arm reached a 24-week viral load under 50 copies if they had no active drugs in the OBR. Respective sub-50-copy rates with one active drug in the OBR were 43%, 43%, and 9%, with two active drugs 53%, 52%, and 19%, and with three or more active drugs 58%, 61%, and 55%.
A key concern about CCR5 antagonist therapy is that it may drive HIV to switch allegiance from the CCR5 coreceptor to CXCR4 or to a dual/mixed tropism status, meaning a person's viral population can use either coreceptor. Research links X4 tropism with advancing HIV disease. A combined analysis of MOTIVATE 1 and 2 showed that substantially more people with treatment failure in the maraviroc arms than in the placebo arms had a tropism switch. And these coreceptor shifts correlated with poorer CD4 gains in the maraviroc arms.
Eighty people in the placebo group had virologic failure without a shift from R5 tropism, and they gained an average 15 CD4 cells. Eighteen people taking once-daily maraviroc and 17 taking the drug twice daily had virologic failure with no coreceptor switch, and they gained an average 61 and 138 CD4 cells. Only 4 people taking placebo had a tropism switch before failure, and they gained an average 67 CD4 cells. In contrast, 31 people in the once-daily maraviroc arm and 32 in the twice-daily arm had a tropism shift before failure, and their average CD4 gains measured only 31 and 32 CD4 cells.
Five people in an AIDS Clinical Trials Group study of vicriviroc had a new cancer diagnosis while taking the drug [3]. Although these cancers could not be directly blamed on the CCR5 antagonist, the finding raised concerns that these agents may somehow promote malignancy. That didn't happen in MOTIVATE 1 and 2. Few people in any treatment arm had a new cancer diagnosis, and there were no more cancers in the maraviroc arms than in the placebo arm.
An FDA advisory panel will consider approval of maraviroc in April.
References
1. Nelson M, Fatkenheur G, Konourina I, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic, ART-experienced patients infected with CCR5-tropic HIV-1 in Europe, Australia, and North America: 24-Week Results. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 104aLB.
2. Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 104bLB.
3. Gulick R, Su Z, Flexner C, et al. ACTG 5211: phase II study of the safety and efficacy of vicriviroc in HIV-infected treatment-experienced subjects. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THLB0217.
|
| |
|
 |
 |
|
|