 |
 |
 |
| |
Atazanavir Use in Pregnancy: a report of 33 cases
|
| |
| |
Reported by Jules Levin
CROI, Feb 2007, Los Angeles
Macky Natha1, Phillip Hay1, Graham Taylor2, Chris Wood3, Liat Sarner4, Laura Cunningham5, John Watson6, Gary
Brook7, Jane Anderson8, Annemiek de Ruiter9 : Perinatal Pregnancy Group London UK
St Georges Healthcare NHS Trust1, Imperial College London2, North Middlesex University Hospital NHS Trust3, Barts and the London NHS
Trust4, Newham University Hospital NHS Trust5, Mayday Healthcare NHS Trust6, North-West London Hospitals NHS Trust7, Homerton University
Hospital NHS Foundation Trust8, Guy’s and St Thomas’ NHS Foundation Trust9
Background
Atazanavir (ATV) has been approved for use in Europe in treatment-experienced HIV patients since March 2004.
However, the use of atazanavir in pregnancy remains unlicensed.
In the developed world, most HAART regimens used during pregnancy include a protease inhibitor. There are limited data on the use of atazanavir in pregnancy.
Atazanavir is labelled category B in pregnancy and there have been concerns about infant hyperbilirubinaemia with use of this drug in pregnancy.
Despite this, atazanavir is increasingly used in pregnancy, either when women conceive on therapy, or when it is initiated in pregnancy due to adherence, resistance or tolerability issues.
Methods
A retrospective case notes review was performed on all women who were receiving atazanavir in pregnancy from nine London HIV units up to February 2007.
Data were collected on maternal demographics, CD4 count, viral load, liver function tests, toxicity and plasma atazanavir concentrations.
Infant data collected included birth weight, serum bilirubin, use of phototherapy and where available, HIV status at 6 months.
Adverse events were also recorded in both mothers and infants.
Results
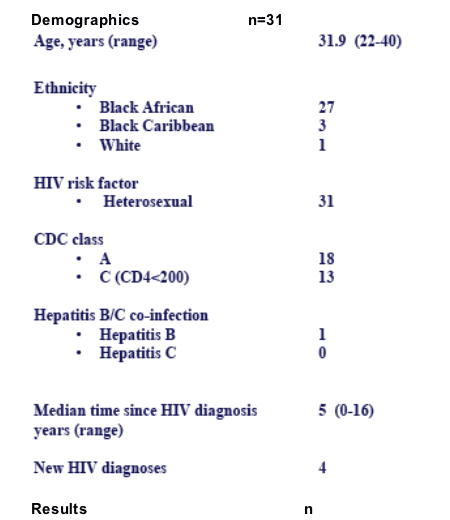
31 women were identified with a total of 33 pregnancies.
There were 2 miscarriages, at 12 and 16 weeks respectively. 1 woman is still pregnant.
All but 2 women were taking the 300mg/100mg ATV/RTV dose.
20 women conceived on ATV. At first antenatal visit, these women had a median VL of 98 c/ml (range <50-101,454 c/ml). Their median CD4 count was 262 (range 61-660) x 109/l.
10 women initiated ATV at a median of 22 (range 5-33) wks gestation.
At first antenatal visit, they all had a VL of <50 c/ml and a median CD4 count of 270 (range 151-930) x 109/l at baseline.
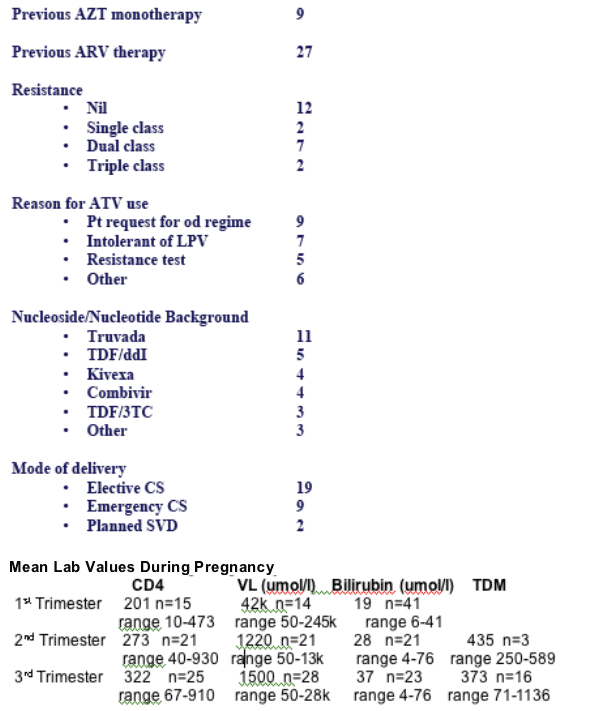
Pre-treatment serum bilirubin and ALT were within normal limits.
At delivery: Median VL and CD4 counts were <50 c/ml (range 50-28189 c/ml) and 269 (range 67-910) x 109/l respectively. 23/30 women had an undetectable viral load.
At delivery: Median maternal bilirubin level was 30 (range 4-76) umol/l [1.8 (range 0.2-4.4) mg/dL] and 23/26 mothers had a raised bilirubin (>17umol/l [>1mg/dL]).
19 mothers had ATV concentrations measured, performed at a median of 30 weeks gestation.
16 were taken in the 3rd trimester and the mean trough ATV concentration was 373 mg/l.
All but two were above the recommended therapeutic concentration of 100 mg/l.
29 infants were born at a median of 38 weeks gestation.
Mean birth weight was 2894g (range 1080-4050g) and median infant bilirubin levels were 71 (range 10-191) umol/l [4.2 (range 0.6-11.2) mg/dL].
3 infants had ATV concentrations measured within 24 hours of birth, showing levels of 189, 129 and 66 mg/l, respectively.
The mother of the last infant stopped all her ARVs 2 weeks before birth.
No infants required phototherapy and no birth defects were detected.
The median time since delivery is 12 months (range 1-26 months) and all infants are thus far HIV uninfected.
AUTHOR CONCLUSIONS
To our knowledge this is the largest reported case series of the use of atazanavir in pregnancy.
Atazanavir was well tolerated in our cohort and most women had good immunological and virological responses.
Maternal plasma levels, where measured, were adequate.
No early infant morbidity as a result of in utero exposure to atazanavir was demonstrated and all infants are thus far HIV uninfected.
|
| |
|
 |
 |
|
|