 |
 |
 |
| |
48-week primary analysis of trial TMC278- C204:
TMC278 demonstrates potent and sustained efficacy in ARV-naive patients
|
| |
| |
A Pozniak, J Morales-Ramirez, L Mohapi,
M Santoscoy, P Chetchotisakd, M Hereygers,
S Vanveggel, M Peeters, B Woodfall and K Boven
Reported by Jules Levin
CROI Feb 28, 2007, LA
Authors concluded:
-- TMC278 demonstrated potent and sustained antiviral efficacy over 48 weeks:
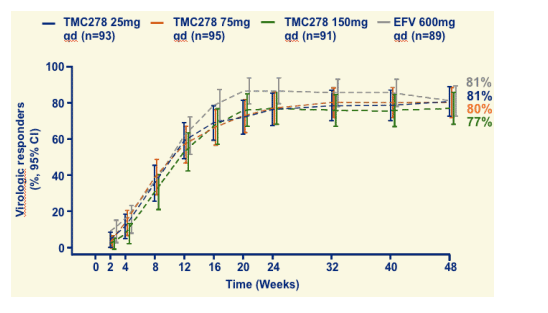
77-81% (TLOVR, NC= F, <50 copies/mL)
-- TMC278 was generally safe and well-tolerated
-- Incidence of rash and nervous system-related events and total
cholesterol/triglycerides were lower with TMC278 than with EFV
-- The 75mg dose, one pill once daily, has been selected for further development in treatment-naive HIV patients
Background
TMC278, a next generation NNRTI, has demonstrated in vitro and in vivo activity against wild-type and NNRTI resistant isolates
TMC278 has a terminal half-life of 45 hours in humans
All doses (25-150mg) of TMC278 significantly reduced viral load in a Phase IIa study in ARV-naive patients2
TMC278-C204 Phase IIb, ARV-naive patients
Almost 400 patients were randomized to efavirenz or 1 of 3 doses of TMC278 (plus 2 nukes), the 75 mg dose has been selected to move ahead with development. It will take two years for this drug to reach availability outside of trials, phase III study should begin by end of 2007.
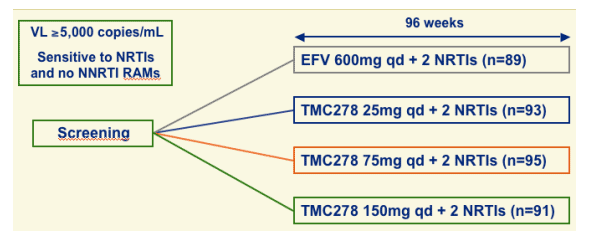
Randomized controlled study.
TMC278 blinded for all 3 dose groups versus open label efavirenz.
Stratification factors
--Investigator-selected NRTI backbone: Combivir (75.3%) or Truvada (24.7%) (given as combination or individual components)
--Region (Asia and Africa; US, Europe and Russia; Latin America)
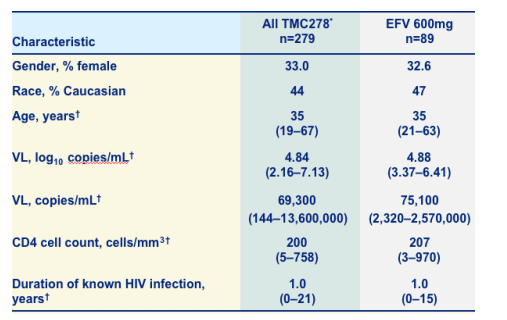
Demographic and baseline characteristics
33% of parients are female
45% Caucasian
average viral load: 4.85 log (70,000 c/ml)
Average CD4 count: 200
Duration of known infection: 1 yr.
Patient disposition at Week 48
Primary efficacy endpoint, ITT population
80% of patients achieved <50 c/ml. There were only 5/6 viral allures in the TMC278 75mg/150mg, and EFV arms. The discontinuation rates were low as well: 5-9 patients for AEs, 4-8 patients for other reasons.
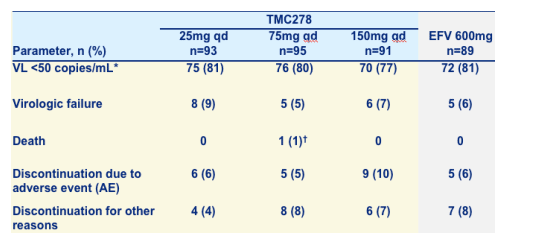
*TLOVR = time to loss of virologic response; NC=F = non-completer = failure; ITT = intent to treat.
Virologic response and loss of response need confirmation with subsequent VL measurement.
Not related to TMC278
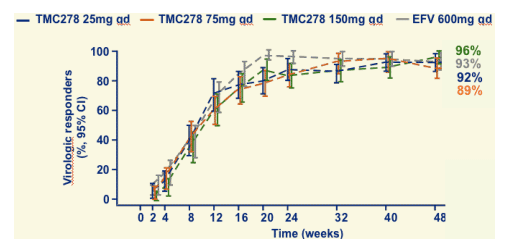
VL <50 copies/mL through 48 weeks (observed)
By observed analyses, 89-96% of patients achieved <50 c/ml.
VL <50 copies/mL through 48 weeks (TLOVR)
Primary efficacy endpoint, ITT population (NC=F)
By ITT analyses, 77-81% of patients achieved <50 c/ml.
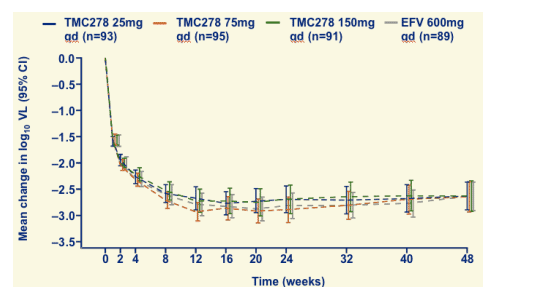
Change in log10 plasma VL through 48 weeks
Viral load declined by about 2.6 log at week 48 for all treatment arms.
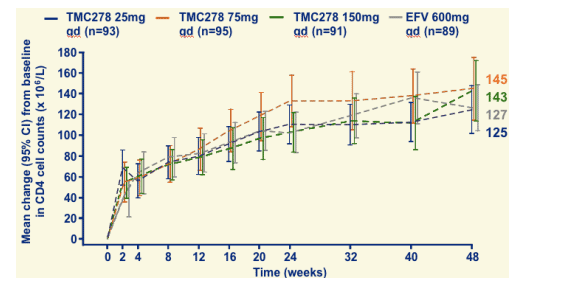
Change in CD4 cell count through 48 weeks
CD4 increased by 130-140 cells.
Most common AEs* at least possibly related to TMC278 or efavirenz
Rates of nausea & headache were about the same for TMC278 & EFV, but there was a lower rate of CNS associated side effects with TMC278 than EFV: dizziness 5.4% vs 27%, somnolence: 3.2% vs 10.1%, vertigo: 1.1% vs 10.1%, abnormal dreams: 1.8% vs 5.6%; and a lower rate of rash was reported: 0.4% vs 5.6%.
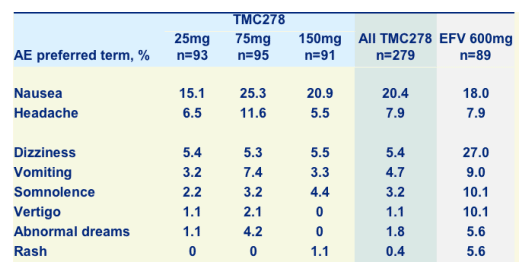
*Occurring in >5% of patients in all TMC278 groups combined or control
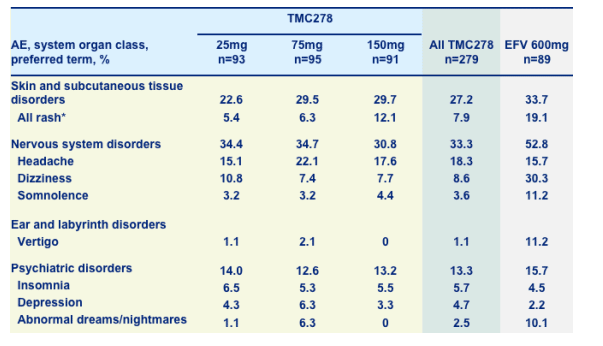
NNRTI class effects, any grade, irrespective of causality
*All rashes were grade 1/2 except one patient with grade 3 rash plus fever (75mg TMC278 group) probably related to dapsone
Skin & subcutaneous tissue disorders: 27% for TMC278, 33% for EFV; all rash*: 7.9% TMC278, 19% EFV.
Nervous system disorders: 33% TMC278, 52% EFV
--headache: 18% TMC278, 16% EFV
--dizziness: 8.6% TMC278, 30.3% EFV
--somnolence: 3.6% TMC278, 11.2% EFV
Ear & labyrinth disorders: vertigo-1.1% TMC278, 11.2% EFV
Psychiatric disorders: 13.3% TMC278, 15.7% EFV
--insomnia: 13.3% TMC278, 15.7% EFV
--depression: 4.7% TMC278, 2.2% EFV
--abnormal dreams/nighmares: 2.5% TMC278, 10.1% EFV
Serious adverse events (SAEs) and grade 3/4 adverse events (AEs)
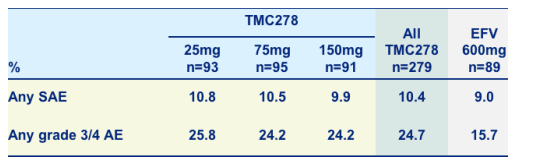
- SAEs at least possibly related to treatment
- TMC278 25mg: 3 patients; 75mg: 0 patients; 150mg: 2 patients and EFV: 1 patient
- One death in TMC278 75mg qd group (not related) due to pneumonia, septic shock
- Difference in G3 and G4 AEs largely due to investigations reported as AE (11.1% in TMC278 and 6.7% in EFV) but no difference in G3 and G4 lab abnormalities
Treatment-emergent grade 3/4 laboratory abnormalities
Rates of grade 3-4 ALT/AST, creatinine, and hemoglobin were low for TMC278 & EFV.
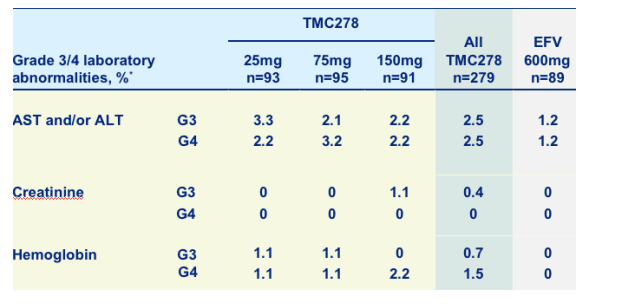
All grade 3/4 lab abnormalities: TMC278 22%, efavirenz 20%
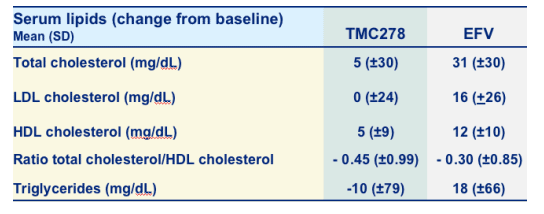
Lipids Laboratory data over time (change from baseline, mg/dL)
Total cholesterol: +5 TMC278, +31 EFV
LDL-cholesterol: 0 TMC278, +16 EFV
HDL-cholesterol: +5 TMC278, +12 EFV
Ratio total chol/HDL chol: -0.45 TMC278, -0.30 EFV
Triglycerides: -10 TMC278, +18 EFV
Endocrine tests-
No clinically relevant changes in endocrine laboratory parameters
|
| |
|
 |
 |
|
|