 |
 |
 |
| |
Adverse Events/Complications of ART in Africa
|
| |
| |
Session 142 - Complications of ART in Resource-Limited Settings
Tuesday, February 26, 2007
Carl J. Fichtenbaum, M.D.
University of Cincinnati College of Medicine
In this poster abstract session were 4 abstracts detailed complications in resource-limited settings. Overall, the results were exactly what one would expect given the availability of antiretroviral medications. In the first abstract, Amoroso and colleagues present data on rates of adverse events leading to treatment discontinuation. It is reassuring that these rates are similar to those observed in the resource rich setting observed years ago with the notable exception that anemia from Zidovudine (AZT) is higher than reported in cohorts from the US and Europe. This may reflect other underlying nutritional issues and higher incidence of anemia.
In the second abstract, Buchacz and colleagues reported results of stored sera tested for lipid levels in persons mostly on d4T or ZDV based NVP regimens. As expected, cholesterol levels were low at initiation of therapy and rose after 24 months with relatively few individuals reaching NCEP definitions of serious lipid disorders. These results parallel cohorts in the US and Europe though the increases seen are less overall again perhaps reflecting differences in dietary factors, body mass index and other genetic factors. The authors conclude the risk for cardiovascular disease is low but probably more time is needed. Cohorts like the D.A.D. study are needed in sub-Saharan Africa and other resource-limited areas to define the true risks of ARV.
In Abstract 791, Peters and colleagues presented data on renal dysfunction, which is common in Ugandan patients with HIV. Reassuringly, renal function improved on NNRTI based therapy with few patients having moderately severe kidney disease (3%). Although many have complained about the use of d4T in this part of the World, it will be interesting to see what happens with more widespread use of tenofovir in these populations with reduced creatinine clearance. Osler and colleagues presented data on lactate levels in South Africa in patients on d4T (Abstract 792). Rates of lactic acidosis and fatalities were lower than reported elsewhere in this region of the World indicating greater awareness (author's note). Curiously, higher weight and weight gain after initiation of ART were more frequently associated with development of symptomatic hyperlactatemia. This study helps inform those in resource-limited settings what to look for and do to avoid and treat elevated lactate levels. The final study in this session focused on anemia after switching from d4T to ZDV. No data on virologic control or opportunistic infections was presented so it's hard to understand the meaning of this data.
789
Antiretroviral-assocated Drug Toxicities Leading to a Switch in Medication: Experience in Uganda, Kenya, and Zambia
Anthony Amoroso*, R Sheneberger, A Edozien, J Fielder, M Etienne, and K Stafford
Univ of Maryland Sch of Med, Baltimore, US
Background: AIDSRelief is a care and treatment program supported by the President's Emergency Plan for AIDS Relief working in 9 countries. The program started treatment of patients with ART in August 2004. Recorded for quality assurance and evaluation are outcomes data, including mortality, lost to follow-up, and ART-associated toxicity leading to a change in treatment. Here we report the ART-associated toxicity reported between August 2004 and June 1, 2006, for 6520 treated patients in Kenya, Zambia, and Uganda.
Methods: A standardized medical record format is used in the 3 countries. Only drug-associated toxicities leading to a clinical decision to change an ART medication are recorded within the medical record and subsequently abstracted into CareWare electronic database. All providers have access to basic chemistry assays, including creatinine, amylase, aminotransferasem alanine aminotransferase, hematocrit or hemoglobin, triglycerides, and blood glucose. The frequencies of associated toxicities were aggregated across the 3 countries regardless of the drug with which the regimen was combined.
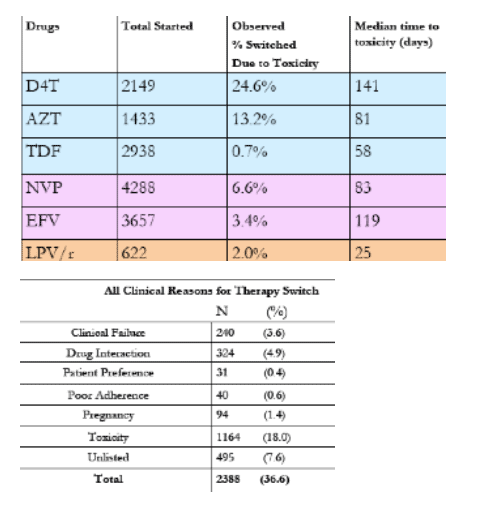
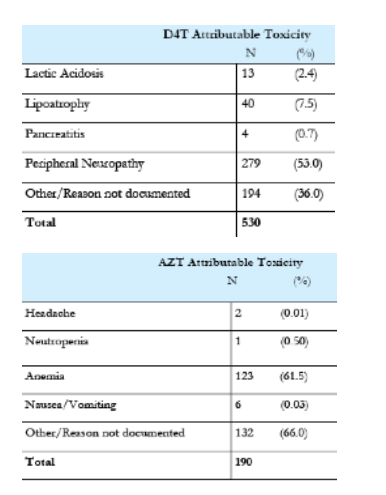
Results: A total of 1007 patients experienced a toxicity that resulted in a drug switch. Of 2149 patient treated with stavudine (d4T), 24% switched because of an associated toxicity, including neuropathy, lipoatrophy, pancreatitis, or lactic acidosis. Of the 1433 patients treated with zidovudine (AZT), 12% switched because of anemia or gastrointestinal intolerance. Of the 2938 treated with tenofovir (TDF), 0.6% switched because of renal insufficiency. Of the 4288 patients treated with nevirapine (NVP), 5% switched because of rash or hepatic toxicity. Of the 3657 patients treated with efavirenz (EFV), 2% switched because of central nervous system intolerance, rash, or hepatic toxicity. Of the 622 patients treated with lopinavir/ritonavir (LPV/r), 1.3% switched because of metabolic abnormalities, liver toxicity, or diarrhea.
Conclusions: Toxicities reported in our program are similar to other published reports in Africa. The comparably low toxicity rates of TDF and EFV support the preferential use of these agents for a "public health" approach over the current predominately used agents. Limitations of the study include an underestimation of toxicities and reliance on provider diagnosis and discretion to change therapy.
790
Changes in Lipid Profile over 24 Months among Adults on First-line HAART in the Home-based AIDS Care Program in Rural Uganda
Kate Buchacz*1, P Weidle1, D Moore2, W Were2, J Mermin2, R Downing2, A Kigozi2, V Ndazima2, and J Brooks1
1CDC, Atlanta, GA, US and 2CDC Uganda, Uganda Virus Res Inst, Entebbe
Background: ART use, particularly with protease inhibitors (PI) and stavudine (4dT), has been linked to dyslipidemia and increased risk of cardiovascular disease in HIV-infected patients in industrialized countries. Its effects on lipid metabolism in patients in Sub-Saharan Africa, where ART programs are rapidly expanding, remain largely unknown.
Methods: From May 2003 to December 2004, 1029 ART-na´ve patients with symptomatic HIV disease or CD4 cell counts ≦250 cells/mm3 initiated HAART in the Home-based AIDS Care (HBAC) program in Tororo, a rural area of Uganda. Non-fasting repository sera from a subset of 596 patients were analyzed for total cholesterol (TC), direct low-density lipopoprotein cholesterol (LDL), direct high-density lipoprotein cholesterol (HDL), and triglyceride (TG) levels prior to HAART and after 12 and 24 months of therapy.
Results: Patients (59% women, median age 39 years, median CD4 cell count 118 cells/mm3, median body mass index 19.4 kg/m2) received d4T plus lamivudine (3TC) with either nevirapine (NVP) (574 patients, 96%) or efavirenz (EFV) (22 patients, 4%). During 24 months of treatment, 12 (2%) patients switched to second-line HAART, which included lopinavir/ritonavir (LPV/r); their observations after the switch were excluded. Single-drug substitutions included zidovudine (AZT) for d4T (136 patients, 23%), and EFV for NVP (36 patients, 6%). Baseline median serum lipid concentrations (mg/dL) were 117 for TC, 49 for LDL, 26 for HDL, and 119 for TG, and were comparable for men and women. Among 480 patients with both baseline and 24-month data, TC increased by a median of 24%, LDL by 54%, HDL by 62%, whereas TC:HDL ratio decreased by 24% and TG decreased by 15% (all changes, t-test p <0.01). At baseline and 24 months, respectively, TC was ≥200 mg/dL for 3% and 11% of patients, LDL was >130 mg/dL for 1% and 6%, HDL was <40 mg/dL for 89% and 42%, and TG were ≥150 mg/dL for 29% and 20%.
Conclusions: Among persons with advanced HIV disease in rural Uganda, elevated TC, LDL, and TG were infrequent before and after 2 years of non-nucleoside reverse transcriptase inhibitor-based HAART. HDL levels increased substantially. The effect of lipid profiles on cardiovascular disease risk in this population is largely unknown; however, the changes we observed after 24 months of HAART appear unlikely to increase the risk of cardiovascular disease.
791
Renal Function Improves among Ugandans on NNRTI-based HAART: 24-Month Follow-up from the Home-based AIDS Care Program in Rural Uganda
Philip Peters*1, D Moore2, J Mermin2, J Brooks1, R Downing2, W Were2, A Kigozi2, K Buchacz1, and P Weidle1
1Natl Ctr for HIV, Viral Hepatitis, STD, and TB Prevention, CDC, Atlanta, GA, US and 2Global AIDS Prgm, CDC Uganda, Uganda Virus Res Inst, Entebbe
Background: Although renal dysfunction is a common complication of advanced HIV disease, little is known about its prevalence in Africa. Moderate renal dysfunction can complicate antiretroviral treatment by requiring dose adjustments for some medications.
Methods: From May 2003 to December 2004, the Home-based AIDS Care (HBAC) program initiated HAART for 1029 ART-naive patients with symptomatic HIV disease or CD4 cell count ≦250 cells/mm3 and estimated creatinine clearance >25 mL/minute. Standard weight-based dosing was administered but not adjusted for renal function. HBAC provides weekly drug delivery, adherence monitoring, and targeted clinic visits. We analyzed serum specimens for creatinine from a subset of 507 patients whose specimens were available from baseline and month 24. Creatinine clearance was estimated by the Cockcroft-Gault equation. The Wilcoxon rank sum and chi-square tests were used to evaluate changes in creatinine levels and to compare rates of moderate renal dysfunction (creatinine ≥1.50 mg/dL).
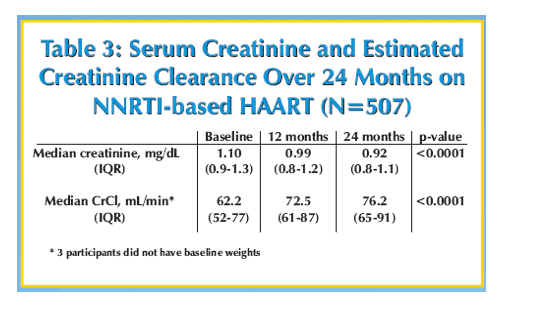
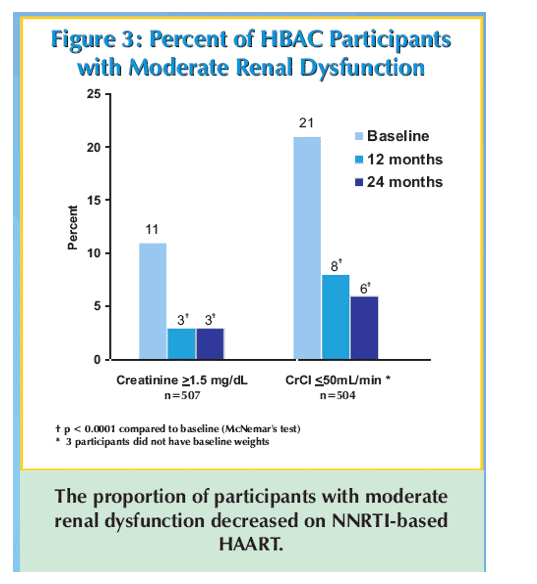
Results: Patients (59% women, median age 39 years, median CD4 cell count 122 cells/mm3, median viral load 245,000 copies/mL, and median weight 54 kg) received stavudine + lamivudine with nevirapine (495 patients, 98%) or efavirenz (12 patients, 2%). Baseline median creatinine was 1.10 mg/dL and 54 (11%) of 507 patients had a creatinine ≥1.50 mg/dL. Median creatinine clearance was 62.5 mL/min and 107 (21%) of 504 patients had creatinine clearance <50 mL/minute. Characteristics associated with moderate renal dysfunction at baseline were age of ≥45 years (p = 0.01) and male gender (p = 0.0001), but not CD4 cell count or viral load. Median creatinine decreased at 12 months to 0.99 mg/dL and at 24 months to 0.92 mg/dL (16% decline from baseline, p <0.0001). At 24 months only 13 (3%) of 507 patients had a creatinine ≥1.50 mg/dL (p <0.0002 vs baseline). After 24 months of follow-up the median creatinine clearance increased to 76.3 mL/minute (22% increase from baseline, p <0.0001) and only 32 (6%) of 504 patients had a creatinine clearance ≦50 mL/minute (p <0.0001 vs baseline). Among patients (n = 54) with moderate renal dysfunction at baseline, creatinine decreased from a median of 1.60 mg/dL to 1.05 mg/dL at 24 months (39% decrease, p <0.0001).
Conclusions: Moderate renal dysfunction was common among this population with advanced HIV disease in rural Uganda, but improved during 2 years of non-nucleoside reverse transcriptase inhibitor-based HAART. Improvements in renal function are most dramatic in patients with moderate renal dysfunction at baseline.
792
Severe Hyperlactatemia Complicating ART with Stavudine First-line Therapy in South Africa: Incidence, Risk Factors, and Outcomes
Meg Osler*1,2, Meg Osler*1,2, D Stead3, K Rebe2,3, K Rebe2,3, A Boulle1,2, A Boulle1,2, G Meintjes2,3, and G Meintjes2,3
1Provincial Dept of Hlth, Cape Town, South Africa; 2Univ of Cape Town, South Africa; and 3GF Jooste Hosp, Cape Town, South Africa
Background: In the public sector ART program in South Africa, the first-line regimen is stavudine (d4T) + lamivudine (3TC) + a non-nucleoside reverse transcriptase inhibitor (NNRTI). This study, undertaken at a referral center for 6 ART clinics, documented the referral rate, risk factors, and outcomes for patients presenting with severe hyperlactatemia (SHL).
Methods: All cases presenting with lactate levels ≥5 mmol/L related to ART from August 2003 to November 2005 were retrospectively reviewed. The cumulative exposure to ART among patients attending the 6 ART clinics was calculated to derive an estimated rate of referral. Associations between risk factors and acute mortality were explored using logistic regression. In a matched case-control study with incidence density sampling, 55 cases from this cohort were matched with 110 controls according to clinic and month starting ART. Conditional logistic regression was used to illicit associations between risk factors and SHL.
Results: There were 73 patients who presented with SHL (69 were females and median age was 32.5 years). Patients had been on ART for a median of 7 months (IQR 5 to 9). During this period there was a cumulative exposure to ART of 7080 patient-years at all 6 ART clinics resulting in an estimated rate of referral for SHL of 10/1000 treatment years. Of the total, 11 patients (15%) died acutely and 2 died subsequently. SHCO3 below 15 mmol/L was the only risk factor consistently associated with acute mortality in univariate and multivariate modeling (OR = 35, p = 0.004, adjusted for age). There were 29 selected cases that rechallenged on zidovudine (AZT)/3TC (all had sHCO3 ≥14.1 mmol/L) were followed for a median of 10 months with lactate monitoring. Only 1 developed recurrent hyperlactatemia. In the case-control study multivariate analysis, a baseline weight >60 kg, female gender and a low nadir CD4 count all increased the risk of acquiring SHL when controlling for age. Compared to people with a baseline weight <60 kg, those between 60 and 74 kg were at 5.1 times higher risk for SHL (95%CI 1.48 to 17.43) while those >75 kg had a 12.2-fold increased risk (95%CI 3.25 to 45.85). The odds ratio for females was 33.6 (95%CI 4.82-234.3) while each additional nadir CD4 cell/mm3 decreased the risk of SHL by 1% (OR = 0.99 95%CI 0.98 to 0.99).
Conclusions: A rate of referral for SHL of 10/1000 treatment years was documented. Female gender, high baseline weight, and low nadir CD4 count were risk factors for SHL. Rechallenge with AZT in selected cases seems safe where lactate can be monitored.
793
Hematologic Changes Associated with Zidovudine after Single-drug Substitution from Stavudine in a Home-based AIDS Care Program in Rural Uganda
Fatu Forna*1, D Moore2, J Mermin2, J Brooks1, W Were2, K Buchacz1, R Downing2, C Borkowf1, and P Weidle1
1CDC, Atlanta, GA, US and 2CDC Uganda
Background: ART improves hematologic abnormalities associated with HIV disease. However, zidovudine (ZDV), which is frequently substituted for stavudine (d4T) when patients develop neuropathy, can cause anemia and leukopenia. We evaluated hematologic changes associated with ZDV following single-drug substitution from d4T in Uganda.
Methods: From May 2003 through December 2004, HIV-infected adults with symptomatic HIV disease or CD4 cell counts ≦250 cells/⊥ received co-trimoxazole and a d4T-containing ART regimen. Through April 2006, we evaluated the effect of substitutions of ZDV for d4T for neuropathy. Abnormal quarterly hematologic parameters were graded according to 2004 DAIDS criteria. We calculated incidence rates (IR) and hazard ratios (HR) of hematologic abnormalities while receiving d4T and after switch to ZDV.
Results: ART was prescribed for 1029 adults (median observation time, 25.6 months/patient). Before starting ART, 266 (26%) patients had a hemoglobin level ≦10 g/dL, 91 (9%) had white blood cell counts ≦2500/mm3, and 139 (14%) had platelets ≦125,000/mm3. While taking d4T, hemoglobin levels declined ≥1 grade from baseline for 83 patients (IR 0.31 of 100 person-months) and were severe grade (<7.5 g/dL) for 19 (0.07/100 person-months). Leukocytes declined ≥1 grade for 86 patients (0.32 of 100 person-months) and were severe grade (<1500/mm3) for 8 (0.03 of 100 person-months). Platelets declined ≥1 grade for 97 patients (0.35 of 100 person-months) and were severe grade (<50,000/mm3) for 11 (0.04 of 100 person-months). ZDV was substituted for d4T for 261 (25%) patients (median observation time on ZDV, 17.3 months per patient). While taking ZDV, hemoglobin levels declined ≥1 grade for 22 patients (0.49 of 100 person-months) and were severe for 6 (0.13 of 100 person-months); leukocytes declined ≥1 grade for 61 patients (1.39 of 100 person-months) and were severe for 2 (0.04 of 100 person-months); and platelets declined ≥1 grade for 16 patients (0.35 of 100 person-months) and were severe for 3 (0.06 of 100 person-months). An increased hazard for leukopenia was associated with receiving ≥6 months of d4T before switch to ZDV (HR, 2.63; 95% confidence interval [CI] 1.51 to 4.60) or a body mass index <18 kg/m2 (HR, 1.81, 95%CI 1.09 to 3.01). Of 261 patients taking ZDV, 13 (5%) switched to another drug for toxicity.
Conclusions: Patients had a slightly higher incidence of anemia and leukopenia after switch from d4T to ZDV but severe hematologic abnormalities were rare. Hematologic toxicity was not a major complication after single-drug substitution from d4T to ZDV in Uganda.
|
| |
|
 |
 |
|
|