 |
 |
 |
| |
Metabolic and Cardiovacular Complications in HIV
|
| |
| |
CROI Report on Session 12-Oral Abstracts, Monday, February 26, 2007
Reported by Carl J. Fichtenbaum, M.D. University of Cincinnati College of Medicine
Metabolic and Cardiovascular Complications of ART (antiretroviral therapy) still plays an important role in shaping when we start antiretroviral therapy and which agents we choose. Today's session had nine presentations of high quality research. In Abstract 37, Baker and colleagues presented data from the FIRST study on non-opportunistic diseases focusing on cardiovascular, renal and liver complications. Their hypothesis was that lower CD4+ count levels would predict outcome. No surprises in this presentation. At low CD4+ opportunistic diseases (OD) are more common and that raising CD4+ helps both OD and non-OD events.
The most surprising result came from Abstract 38 presented by Richard Haubrich and colleagues for the ACTG A5142 team. In this metabolic substudy they report that lipoatrophy was significantly more common in subjects receiving efavirenz based therapy compared to lopinavir/ritonavir based therapy. Of note, nucleosides used in this study were balanced and did not account for the differences. So, why this surprising finding? There are several possibilities: first, efavirenz may have detrimental effects on adipocytes. There is little data on NNRTI effects on mitochondrial function. Second, lopinavir/ritonavir may have a protective effect sparing adipocytes. One possible theory is that LPV/r may have independent anti-inflammatory effects that decrease inflammation in the adipocytes leading to less fat loss. One clue along these lines is the consistently higher increases in CD4+ counts with the use of LPV/r compared to NNRTIs across a number of studies. The other possibility is that LPV/r leads to increase fat gain thereby masking the loss of fat better than Efavirenz. Clearly, much work needs to be done to tease out the meaning of these findings. I would not change my practice of prescribing based upon this study alone. Clearly, this is a complicated field and what represents "optimalÓ antiretroviral therapy is still a contentious issue.
In Abstract 39, Wohl and colleagues presented data on a placebo-controlled study demonstrating a benefit of ezitimibe (zetia¨), a cholesterol absorption inhibitor. This very nice clean study tells us the benefit of ezitimibe is similar to that seen in non-HIV infected persons. This may be a useful adjunctive therapy in persons with elevated LDL cholesterol.
In Abstract 40, Carey and colleagues presented 24 week data on Poly-L-lactic acid for the treatment of facial lipoatrophy using spiral CT to quantify volume. Although subjects reported improvement and better quality of life, there was no difference when quantitated by CT. Disappointing results that could suggest that there was a technical problem in delivering the treatment correctly; CT is not a good test to measure outcome; or that PLA just doesn't work well. This should give us pause before using this therapy widely at this point.
In Abstract 41, Phillips and colleagues presented further information on Cardiovascular events in the SMART study. This really confirms prior presentations showing that stopping therapy was associated with higher risk of CV events. Overall, number of events were small in both groups making conclusions difficult. It may be that inflammation as a result of uncontrolled HIV may stimulate activation of plaques leading to events. One concern is the technical definition and standardization of CV events by ECG. We'll need more data to interpret the occurrence of 18 silent MIs.
In Abstract 42, Grace McComsey and colleagues presented data on a randomized trial of alendronate for osteopenia and osteoporosis in HIV. They confirmed that alendronate is useful and the benefit is comparable to that seen in non-HIV infected persons. Unfortunately, this study was not designed to answer the real question which is whether we should be doing DEXA scans on all of our patients; how often they should be done; and whether we should be treating patients with HIV and osteopenia differently from the general population.
In Abstract 43, Grinspoon and colleagues presented data on using d4T in healthy HIV seronegative adults. They demonstrated an immediate effect on glucose metabolism after just 4 weeks of use. While the mechanism may not be entirely clear, the implications are. Worldwide, d4T is very commonly used in resource poor settings. This is a recipe for disaster. In resource rich settings, d4T is rarely used these days because of the many side effects including those demonstrated eloquently by Grinspoon. This is more evidence that should inform policy makers to change use of d4T in the World.
In Abstract 44LB, Cameron and colleagues presented further data indicating that LPV/r may preserve fat depots peripherally. In this study where everyone received LPV/r induction followed by LPV/r monotherapy versus Efavirenz plus NRTIs, the authors concluded that LPV/r monotherapy preserves fat. Unfortunately, this study is complicated by the use of ZDV/3TC in the efavirenz arms. It may be that fat loss was driven by the NRTIs. However, it is still curious that LPV/r resulted in improvement in fat from baseline. This may simply represent restoration of health in persons with advanced HIV infection. So, this story is still complicated and needs to be teased out further.
Finally, in Abstract 45LB, Grinspoon and colleagues presented results of a large randomized study of TH9507, a growth hormone releasing factor analog. In this study, visceral lipohypertrophy was reduced by 20% with use of the GHRF analog. It was a short term result at 24 weeks. What we'll need is longer follow up and data on how fast lipohypertrophy returns once therapy is stopped. Encouragingly there were few adverse effects and no great effect on glucose levels.
Abstract 37
HIV-related Immune Suppression after ART Predicts Risk of Non-opportunistic Diseases: Results from the FIRST Study
Jason Baker*1,2, Jason Baker*1,2, G Peng1, J Rapkin1, D Abrams3, M Silverberg4, W Cavert1, R MacArthur5, W Henry1,2, W Henry1,2, J Neaton1, and Terry Beirn Community Prgms for Clin Res on AIDS (CPCRA)
1Univ of Minnesota, Minneapolis, US; 2Hennepin County Med Ctr, Minneapolis, MN, US; 3Univ of California, San Francisco, US; 4Kaiser Permanente, Oakland, CA, US; and 5Wayne State Univ, Detroit, MI, US
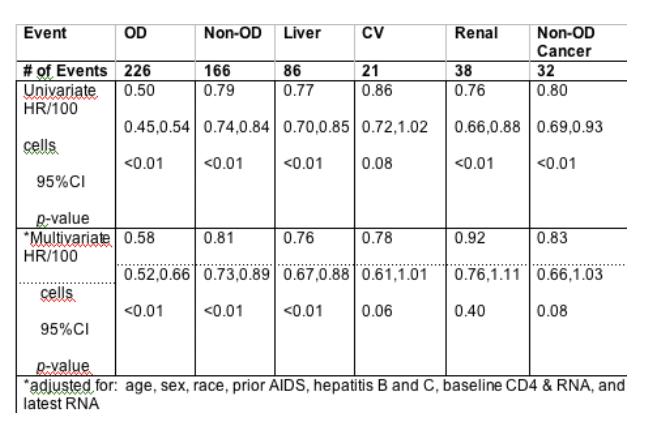
Background: Observational data suggest that the risk of death from non-opportunistic diseases (OD) during HIV infection may be related to immunosuppression. The SMART study showed that episodic ART led to increased risk of cardiovascular (CV), renal, and liver complications compared to continuous ART. The association of HIV-related immune suppression with specific non-fatal and fatal non-OD events after ART has not been well described. We compared the association between latest CD4 level (prior to event) and non-OD events (liver, CV, renal, cancer) using follow-up data from FIRST (substudy CPCRA 058).
Methods: In FIRST, ART-na•ve patients were randomized to 1 of 3 ART strategies (non-nucleoside reverse transcriptase inhibitors [NNRTI] + NRTI (N=463), protease inhibitors [PI] + NRTI (N=470), and 3-class therapy (N=464)). Fatal and non-fatal OD and non-OD events were collected over a 5-year median follow-up and reviewed by an Endpoint Committee. OD events were defined by modified CDC AIDS criteria. Non-OD events included: liver (cirrhosis and grade 4 transaminitis); CV (myocardial infarction, stroke, coronary artery disease requiring surgery or death from CAD); renal (end-stage renal disease, renal insufficiency or death from CKD); non-OD cancers (malignancy excluding non-Hodgkin's lymphoma and Kaposi's sarcoma). Cox models examined the association of OD and non-OD events with latest (time-updated) CD4 count (hazard ratio [HR] represent the difference in risk for a 100 cell higher CD4 count).
Results: Of 1397 persons randomized in FIRST, median entry CD4 was 163 cells/mL, with an average increase of 238 cells/mL during follow-up; CD4 changes and OD rates did not vary by treatment. The baseline viral load was 5.3 log copies/mL. By month 32 of follow up 55% remained with undetectable viral loads. Univariate and multivariate associations with latest CD4 levels are described (table). HR for non-OD events, combined and individually, indicate risk of event is reduced when CD4 count is higher. In individuals with CD4+<200 cells/cu mm the rate of OD events was 14 per 100 pyr and for Non-OD events it was 5 per 100 pyr.
Conclusions: Higher, latest CD4 count was associated with a reduced risk of non-OD events (liver, CV, renal, and cancer), though to a lesser degree than with OD events. These data suggest that treatment strategies minimizing the time spent at lower CD4 counts will prevent both non-OD and OD events. Liver, CV, renal and cancer events account for more M & M than OD events at CD4>200.
Abstract 38
Metabolic Outcomes of ACTG 5142: A Prospective, Randomized, Phase III Trial of NRTI-, PI-, and NNRTI-sparing Regimens for Initial Treatment of HIV-1 Infection
Richard H. Haubrich*1, S Riddler2, G DiRienzo3, L Komarow3, W Powderly4, K Garren5, T George6, J Rooney7, J Mellors2, D Havlir8, and the AIDS Clinical Trials Group 5142 Study Team
1Univ of California, San Diego, US; 2Univ of Pittsburgh, PA, US; 3Harvard Sch of Publ Hlth, Statistical and Data Analysis Ctr, Boston, MA, US; 4Univ Coll Dublin, Ireland; 5Abbott Labs, Abbott Park, IL, US; 6Bristol-Myers Squibb, Plainsboro, NJ, US; 7Gilead Sci, Foster City, CA, US; and 8Univ of California, San Francisco, US
Background: The metabolic effects of LPV- or EFV-based regimens + 2 NRTI have not been compared nor has the role of an NRTI-sparing regimen in preventing lipoatrophy been tested.
Methods: Open-label, randomized trial comparing class-sparing regimens for na•ve subjects: LPV+EFV (LPV/EFV) vs. LPV+2NRTI (LPV; soft-gel BID) vs. EFV+2NRTI (EFV). NRTIs were selected before randomization from ZDV, d4T XR or TDF (each plus 3TC). Metabolic objectives included evaluation of changes in fat (DEXA) and fasting lipids. DEXA and lipids were performed at baseline, 48 and 96 weeks. Lipoatrophy was defined as ≥20% loss of limb fat from baseline. All analyses were ITT without adjustment for multiple comparisons or regimen changes. Pairwise comparisons used non-parametric tests.
Results: 753 enrolled subjects (median CD4 182 cells/mm3, median HIV-1 RNA 100,000 copies/ml) were followed for a median 112 weeks. NRTI choice was ZDV 42%, d4T XR 24%, and TDF 34%. Median baseline values were not different by arm: trunk fat 8.2 kg, extremity fat 7.0 kg, total cholesterol (TC) 154 mg/dl, HDL 35 mg/dl, non-HDL 117 mg/dl and triglycerides (TG) 115 mg/dl. By week 96, 10, 12 and 26% of EFV, LPV and LPV/EFV subjects used a lipid-lowering agent. Week 96 results (below) were similar to other time points.
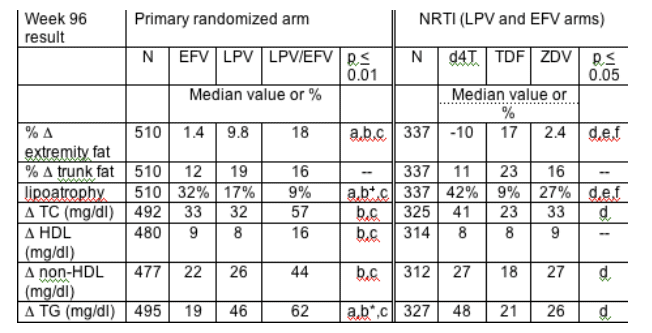
Pairwise comparisons: a=EFV vs LPV; b=LPV vs LPV/EFV; c=EFV vs LPV/EFV; d=d4T vs TDF; e=TDF vs ZDV; f=ZDV vs d4T. b+=0.023,b*=0.030; If not listed, p>0.05.
Lipoatrophy in the EFV or LPV + NRTI was predominately seen in the d4T- or ZDV-containing regimens; there was no significant difference (p>0.5) in lipoatrophy between TDF- containing and NRTI-sparing regimens.
Conclusions: A NRTI-sparing regimen (LPV/EFV) increased lipids significantly more than EFV or LPV + 2 NRTI regimens. TG increases were also greater in LPV compared to EFV + NRTI regimens, but cholesterol changes were not significantly different. Compared to EFV, LPV had less lipoatrophy when given with NRTI. The frequency of lipoatrophy was lowest in NRTI-sparing and TDF-containing regimens.
39
Ezetimibe's Effects on the LDL Cholesterol Levels of HIV-infected Patients Receiving HAART
David Wohl*1, P Hsue2, S Richard1, A Schnell2, S Napravnik1, R Simpson1, J Keys1, and D Waters2
1Univ of North Carolina at Chapel Hill, US and 2Univ of California, San Francisco, US
Background: Ezetimibe, a novel agent that inhibits the intestinal absorption of dietary and biliary cholesterol, is approved for use with statins for LDL cholesterol (LDL-C) reduction. Its safety and efficacy in HIV+ patients is not known. Ezetimibe decreases absorption of cholesterol by 50% resulting in ~ 10-20% reduction in LDL cholesterol when used alone and up to 55% when combined with a statin. Negredo et al. previously presented data on 19 subjects on HAART treated with Ezetimibe with a 15% reduction in LDL cholesterol.
Methods: Randomized, double-blind, placebo-controlled, 2-period cross-over trial to study the safety and effect of ezetimibe in lowering LDL-C in HIV+ patients on stable HAART but no lipid-lowering therapy with LDL-C ≥75 mg/dL and triglycerides (TG) <800 mg/dL. All subjects had to have a CD4+ count > 100 cells/cu mm and HIV viral load < 10,000 copies/mL. All were randomized to ezetimibe 10 mg daily (N=25) or placebo (N=23) for 6 weeks followed by a 2-week washout and then 6 weeks of treatment with the alternative study assignment. Fasting lipid panel (8 hours) including direct LDL-C and chemistries were evaluated at entry and weeks 6, 8, and 14. Differences in percent change of LDL-C cholesterol (%Ć LDL-C) from pre- to post-intervention between ezetimibe and placebo were compared using generalized linear models with a normal probability distribution fit with generalized estimation equations to account for repeated measures. We used an ITT - LOCF analysis.
Results: We enrolled 48 subjects, of whom 23% were women, 48% African American, median age = 46 years (IQR 43, 51), median CD4 = 555/mm3 (IQR 447, 783), and all but 1 subject had HIV RNA <400 copies/mL. We randomized 25 to ezetimibe followed by placebo and 23 to placebo followed by ezetimibe. At pre-intervention and following washout the median LDL-C was 121 mg/dL (101, 152) and 123 mg/dL, p = 0.70. The median %Ć LDL-C after ezetimibe treatment was -12% (IQR -23%, 1%), and after placebo was 3% (IQR -6%, 17%), p = 0.03. 35% of patients had at least a 17% drop in LDL-C after 6 weeks of ezetimibe. The difference in %Ć LDL-C comparing ezetimibe to placebo was -12% (95%CI -22%, -2%), p = 0.02. Treatment period did not affect these results (p = 0.85). There were no significant changes in HDL-C or TG observed. Of the 5 subjects who discontinued the study drug prematurely, 2 were on ezetimibe (1 grade-3 LFT in subject with grade-1 LFT at entry, 1 epigastric pain) vs 3 on placebo (1 grade-2 LFT, 1 headache and nausea, 1 neuropathy); 2 other subjects had new LFT increases (grade 2), both while on placebo. 5 additional patients had non-treatment limiting grade 2 or higher AEs (2 with headache, one in each arm); 1 diarrhea (EZT arm); and 1 with chest pain (EZT).
Conclusions: Ezetimibe alone led to significant and clinically meaningful declines in LDL-C (12%; 2-22% 95 CI) and was well-tolerated. This trial, the first placebo-controlled study of ezetimibe in HIV+ patients, suggests a role for ezetimibe in the management of elevated LDL-C in patients with HIV. Further, for patients unable to take a statin, monotherapy with ezetimibe may be an option.
40
Poly-L-lactic Acid Injections for Facial Lipoatrophy: A Randomized, Multicenter Trial
Dianne Carey*1, D Baker2, N Easey3, K Petoumenos1, S Emery1, J Chuah4, K Machon5, G Rogers6, D Cooper1,3, D Cooper1,3, A Carr3, and for the Facial Lipoatrophy Study in HIV (FLASH) Investigators
1Natl Ctr for HIV Epidemiology and Clin Res, Univ of New South Wales, Australia; 2407 Doctors, Sydney, Australia; 3St Vincent's Hosp, Sydney, Australia; 4Gold Coast Sexual Hlth Clin, Miami, Australia; 5Natl Assn of People with HIV/AIDS, Sydney, Australia; and 6Secretariat of the Pacific Community, Noumea, New Caledonia
Background: Facial lipoatrophy is disfiguring, can stigmatize, and can affect self-esteem, quality of life, and adherence to ART. Small studies have found poly-L-lactic acid (PLA) injections to have acceptable safety and efficacy over 48 weeks, but no study was randomized and also included objective endpoints. The authors state that facial lipoatrophy affects 50-70% of adults on NRTI+PI therapy (seems a bit high, CJF comment). The purpose of this study was to test the efficacy, safety and tolerability of sc injections of PLA. They hypothesized that recipients of PLA would have >25% increase in facial volume by CT scan.
Methods: HIV+ adults with moderate/severe facial lipoatrophy and lipoatrophy at one other body site were randomised to 4 open-label poly-L-lactic acid treatments (5 mL per cheek) every 2 weeks from week 0 (IMM; n = 51), or 4 poly-L-lactic acid treatments after week 24 (DEF; n = 50). Subjects had to be >18 years old; be on HAART for 3 months or more; and were excluded if they were using steroids, growth hormone. The primary endpoint was mean change from baseline in facial soft tissue volume (upper limit at mid-orbit; lower limit at angle of mandible) by spiral computed tomography (CT). Spiral CT used standard bony landmarks. The study had 80% power to detect a 25% difference in change in facial soft tissue volume between groups at week 24 by intention-to-treat analysis.
Results: Mean changes in facial soft tissue volume at week 24 were 0 cm3 (-14,14)in the IMM group and -10 cm3 (-22,1) in the DEF group (between-group difference, 10 cm3 [95%CI -7, +28]; p = 0.24; +3%). Relative to the DEF group, the IMM group had a significantly greater between-group change in subcutaneous soft-tissue depth just inferior to the malar bone (2.2 mm; 95%CI 1.6, 2.9; p <0.0001; +19%) and just superior to the nasolabial folds (1.0 mm; 95%CI, 0.3, 1.6; p = 0.003; +4%), but not in untreated areas (mid-orbit, p = 0.9; angle of mandible; p = 0.21). Subjective facial lipoatrophy severity assessed by patient and physician improved significantly in IMM participants (p <0.0001). There was no change in peripheral fat mass (p = 0.63), total fat mass (p = 0.29), or lean body mass (p = 0.28) by DEXA. Poly-L-lactic acid treatment adherence was 99%. Poly-L-lactic acid treatment did not significantly impact HIV viral load (p = 0.57), CD4+ count (p = 0.54), or ART adherence (p = 0.70). The most common procedure/product adverse events were pain or discomfort (any grade, 79%), edema (66%; ≥25 mm diameter, 29%) and erythema (56%; ≥25 mm diameter, 22%). Median duration of treatment-related adverse events was 2 (IQR 1 to 3) days. No poly-L-lactic acid-related adverse event was dose-limiting or delayed treatment. Of 4 severe adverse events reported in 3 subjects, none was attributed to poly-L-lactic acid. In the end, 8% assigned to immediate PLA compared to 5% on deferred arm had an increase in facial volume by CT, P=NS at 24 weeks.
Conclusions: Although 4 facial injections of poly-L-lactic acid over 6 weeks in HIV-infected adults with facial lipoatrophy did not increase facial soft tissue volume overall, but prevented deterioration in injected areas. Poly-L-lactic acid injections were administered safely and were well tolerated. Objectively assessed relative increases in cheek subcutaneous thickness were less than reported in other studies.
41
Interruption of ART and Risk of Cardiovascular Disease: Findings from SMART
Andrew Phillips*1, A Carr2, J Neuhaus1, F Visnegarwala3, R Prineas4, W Burman5, I Williams6, F Drummond7, D Duprez1, J Lundgren8, and others for the SMART Study Group
1Univ of Minnesota, Minneapolis, US; 2St Vincent's Hosp, Univ New South Wales, Sydney, Australia; 3Baylor Coll of Med, Houston, TX, US; 4Univ of Wake Forest Sch of Med, Winston-Salem, NC, US; 5Denver Publ Hlth, CO, US; 6Royal Free and Univ Coll London Med Sch, UK; 7Natl Ctr for HIV Epidemiology and Clin Res, Sydney, Australia; and 8Hvidovre Hosp, Copenhagen, Denmark
Background: SMART compared strategies of continuous (viral suppression arm;VS) and CD4-guided (drug conservation arm; DC; off ART when >350 and (re-)start when <250) ART. Patients in DC arm deferred/interrupted ART at baseline; those in VS arm started/continued ART. We compared risk of cardiovascular disease (CVD) events and lipid changes in the 2 arms.
Methods: We used Cox models to assess the association between treatment arm and CVD risk and to study this effect in various subgroups, in particular those relating to specific drugs/classes used at baseline. Mean changes in lipids were assessed between baseline and year 1 (not clear if these were all fasting, CJF comment).
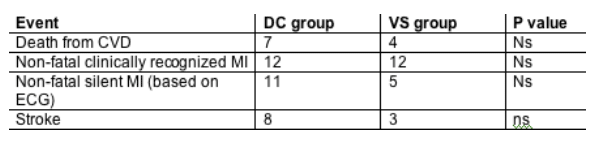
Results: We randomized 5472 patients, of whom 79 (1.4%) developed major CVD events. Note that 4% of the overall cohort had pre-exisiting cardiovascular disease and 40% smokers. The hazard ratio of CAD events (HR [DC/VS]) was 1.57 (95%CI 1.00 to 2.46, p = 0.05). For the subset (84%) on ART at baseline (43% non-nucleoside reverse transcriptase inhibitor [NNRTI] and not protease inhibitor [PI], 45% on PI) the HR (DC/VS) was 1.37 (0.85 to 2.21, p = 0.20), while for those off ART at baseline (16%, 5% na•ve) it was 4.41 (0.94 to 20.8, p = 0.06) (interaction p = 0.16). There was no evidence that currently being off ART (time-updated covariate) was associated with increased CVD risk (HR for being on ART vs off 0.91, 0.57 to 1.47, p = 0.70), nor was higher current viral load associated with increased CVD risk. Among those on ART at baseline, the HR (DC/VS) was higher in those on nucleoside-only (1.78) or NNRTI (2.07) regimens compared with those on a PI (1.00) (interaction p = 0.37). The HR was most marked in those on nevirapine at baseline (HR 9.29, 1.19 to 72.6, interaction p = 0.05). The HR for CVD events per additional year of exposure to the PI was consistent with that found in the DAD study in the VS arm (HR 1.17, 1.03 to 1.33) but not in the DC arm (HR 1.02, interaction p = 0.07), suggesting possibly reduced relevance of cumulative drug use in those who have interrupted. Total, LDL, and HDL cholesterol were reduced in those DC patients who interrupted ART, leading to a net unfavorable change in total/HDL-cholesterol ratio, again particularly in those on nucleoside-only (mean change in ratio from baseline +0.43) and nevirapine regimens (+0.58).Total cholesterol declined significantly more in the DC arm (median -22 mg/dL) vs. the VS arm (median -10 mg/dL).
Conclusions: A borderline significant excess risk of CVD was observed in DC compared to VS patients in SMART. There was no evidence that interruption immediately increases risk of CVD, but longer-term consequences cannot be excluded. On balance, lipid changes were unfavorable after interruption in DC patients, and the extent of this differed according to baseline ART regimen.
Additional data from Abstract:
42
Alendronate with Calcium and Vitamin D Supplementation Is Superior to Calcium and Vitamin D Alone in the Management of Decreased Bone Mineral Density in HIV-infected patients: Results of ACTG 5163
Grace A McComsey*1, M Kendall2, P Tebas3, S Swindells4, E Hogg5, B Alston-Smith6, C Suckow7, G Gopalakrishnan8, C Benson9, D Wohl10, and AIDS Clinical Trials Group ACTG 5163
1Case Western Reserve Univ, Cleveland, OH, US; 2Statistical & Data Analysis Ctr, Harvard Sch of Publ Hlth, Boston, MA, US; 3Univ of Pennsylvania, Philadelphia, US; 4Univ of Nebraska Med Ctr, Omaha, US; 5Social & Sci Systems, Silver Spring, MD, US; 6NIAID, NIH, Bethesda, MD, US; 7Frontier Sci & Tech Res Fndn, Amherst, NY, US; 8Brown Med Sch, Providence, RI, US; 9Univ of California, San Diego, US; and 10Univ of North Carolina at Chapel Hill, US
Background: Decreased bone mineral density is prevalent in HIV-infected patients. Bisphosphonates, potent inhibitors of bone resorption, are currently the mainstay of treatment for postmenopausal and male osteoporosis in the HIV uninfected; however, their efficacy and safety in HIV-infected patients remain unclear.
Methods: A5163 was a prospective, randomized, placebo-controlled multicenter trial to evaluate the effectiveness of calcium and vitamin D supplementation with or without once-weekly alendronate (70 mg) in improving bone mineral density in HIV-infected individuals with lumbar spine t-scores ˛ -1.5 (after 48 weeks duration). Additional criteria included CD4 ≥100 cells/cu mm; HIV viral load ˛ 5,000 copies/mL; excluded if low vitamin D levels <15 IU/L. The study was powered to detect differences of 3.5% between arms and to evaluate moderate effects of gender in the response to therapy. All DEXA scans were analyzed centrally, blinded by arm.
Results: The 82 patients enrolled (42 to alendronate; 40 to placebo) were 71% males, 77% white, with a baseline median age of 48 years. Median CD4 was 469 cells/mm3 and 91% had HIV RNA <400 copies/mL. Median baseline lumbar spine t-score was -2.1. Compared with calcium/vitamin D, alendronate + calcium/vitamin D resulted in improvements in lumbar spine (3.38% vs 1.10%, p = 0.03), total hip (3.95% vs 1.31%, p = 0.004), and trochanter (4.52% vs 0.72%, p = 0.03), but not femoral neck (2.21% vs 1.24%, p = 0.35). There was at least a trend toward increase in the bone mineral density values in calcium/vitamin D at lumbar spine, total hip and femoral neck, with p = 0.08, 0.03, and 0.07 respectively, compared to baseline. Black race was associated with a smaller change from baseline in bone mineral density of lumbar spine with alendronate. There were no apparent gender differences in the responses to therapy. Alendronate was well tolerated, without significant adverse events. Note that: T scores were worse in the alendronate arm at baseline -2.15 vs. -1.95 for Lumbar spine BMD.
Conclusions: The results demonstrate that once-weekly alendronate is safe and efficacious in the treatment of decreased bone mineral density in HIV-infected patients. Vitamin D and calcium alone is associated with modest improvements in bone mineral density.
43
Effects of a NRTI, Stavudine, on Insulin Sensitivity and Mitochondrial Function in Muscle of Healthy Adults
A Fleischman1, S Johnsen1,2, S Johnsen1,2, D Systrom1, M Hrovat1, C Farrar1, W Frontera1,3, W Frontera1,3, K Fitch1, M Torriani1, H Cote4, and Steven Grinspoon*1
1Massachusetts Gen Hosp, Harvard Med Sch, Boston, US; 2Skejby Univ Hosp, Aarhus, Denmark; 3Spaulding Rehabilitation Hosp and Brigham and Women's Hosp, Boston, MA, US; and 4Univ of British Columbia, Vancouver, Canada
Background: Data for individuals at risk for type 2 diabetes mellitus (DM) and for those with HIV infection, suggest that mitochondrial dysfunction may contribute to the development of insulin resistance. Nucleoside reverse transcriptase inhibitors (NRTI), specifically stavudine (d4T), are known to alter mitochondrial function in HIV-infected individuals, but effects may be confounded by other factors. The direct effects on muscle have not been investigated. The current study was designed to test the hypothesis that the use of d4T in a double-blind, placebo-controlled trial in healthy humans results in alteration of muscle mitochondrial function and insulin sensitivity.
Methods: We enrolled in the study 16 healthy participants without personal or family history of diabetes mellitus. Subjects were randomized to receive d4T 30 to 40 mg twice a day, or placebo for 1 month. Insulin sensitivity was assessed by hyperinsulinemic euglycemic clamp, muscle mitochondrial content was determined, and mitochondrial function investigated by 31P spectroscopy of phosphocreatine recovery rates. ANOVA was utilized to detect differences between placebo- and d4T-treated participants. Matched pairs analyses were performed for within group changes.
Results: The study is closed to enrollment and the data presented are final. In the d4T-treated subjects, insulin sensitivity by hyperinsulinemic euglycemic clamp was significantly reduced after 1-month exposure compared to placebo (-0.8±0.5 vs +0.7±0.3 mg/kg/minute, p = 0.04, d4T vs placebo, respectively). In addition, muscle biopsy specimens in the d4T-treated group showed significant reduction in mitochondrial mtDNA/nuclear DNA (-52%, p = 0.004), with no change in placebo-treated subjects (+8%, p = 0.9). The 31P magnetic resonance spectroscopy studies of mitochondrial function, correlated with insulin sensitivity measures (r2 = 0.5, p = 0.008).
Conclusions: This is the first study to demonstrate that d4T causes alterations in muscle mitochondrial content and insulin sensitivity in healthy individuals. The current study supports the hypothesis that alterations in mitochondrial function in muscle contribute to the development of insulin resistance in non HIV-infected patients and also demonstrates an important mechanism of toxicity in HIV-infected patients.
44
Significant Sparing of Peripheral Lipoatrophy by HIV Treatment with LPV/r + ZDV/3TC Induction followed by LPV/r Monotherapy Compared with EFV + ZDV/3TC
DW Cameron*1, B da Silva2, J Arribas3, F Pulido4, H Katner5, K Wikstrom2, M Woulfe2, K Niemi2, M King2, and G Hanna2
1Univ of Ottawa at The Ottawa Hosp, Canada; 2Abbott Labs, Abbott Park, IL, US; 3Hosp La Paz, Madrid, Spain; 4Hosp Univ Doce de Octubre, Madrid, Spain; and 5Mercer Univ Sch of Med, Macon, Georgia, US
Background: Peripheral lipoatrophy is associated with nucleoside analogue ART, but the contribution of protease inhibitors alone to lipodystrophy remains unclear. We assessed body fat changes and potential mediators in a randomized, controlled trial of lopinavir/ritonavir (LPV/r)+zidovudine/lamivudine (ZDV/3TC) induction followed by LPV/r monotherapy, vs efavirenz (EFV)+ZDV/3TC for 96 weeks of treatment.
Methods: We randomized 155 ART-naive HIV+ subjects to LPV/r+ZDV/3TC induction for 24 to 48 weeks followed by LPV/r monotherapy (LPV/r arm, n = 104), or EFV+ZDV/3TC (EFV arm, n = 51). Subjects were followed for 96 weeks with DEXA scans every 24 weeks. Lipoatrophy (>20% limb fat loss) and lipohypertrophy (>20% trunk fat gain) were assessed. Baseline serum lipids and other metabolic parameters were evaluated for association with percent changes in limb or trunk fat among subjects completing 96 weeks of treatment.
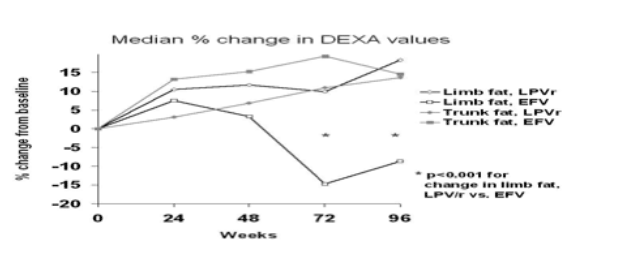
Results: In the LPV/r arm, 74 subjects (71%) and, in the EFV arm, 32 (63%) had serial DEXA scans available to 96 weeks. No baseline differences in DEXA measurements were observed (mean trunk fat: 9.3 kg LPV/r, 8.3 kg EFV, p = 0.46; mean limb fat 8.2 kg LPV/r, 7.4 kg EFV, p = 0.45). A significant difference in limb fat change from baseline was observed (median +18% LPV/r, -9% EFV, p <0.001 between groups) at week 96, while trunk fat changes were similar (+14% LPV/r, +15% EFV). Lipoatrophy was observed in 5% in the LPV/r arm and 34% in the EFV arm (p <0.001) and lipohypertrophy was observed in 45% and 44%, respectively (p >0.99); 0% (LPV/r arm) and 16% (EFV arm) had both lipoatrophy and lipohypertrophy. Baseline and changes from baseline in lipids and metabolic parameters were comparable between treatment groups, except for a higher median triglyceride increase in the LPV/r group (+0.67 vs +0.47 mM/L, p = 0.02). Subjects with low baseline CD4 counts were more likely to have limb fat increases. Age, other baseline demographics, and levels of blood lipids and TNF soluble receptors 1 and 2 were not associated with limb or trunk fat changes. The difference between treatment groups in limb fat changes remained significant after adjusting for baseline CD4 count (p <0.001). There was no statistically significant change in peripheral blood mononuclear cell mtDNA in either group.
Conclusions: Treatment with LPV/r monotherapy (compared with EFV+ZDV/3TC) was significantly and independently associated with sparing of peripheral lipoatrophy.
45
Effects of TH9507, a Growth Hormone Releasing Factor Analog, on HIV-associated Abdominal Fat Accumulation: A Multicenter, Double-blind Placebo-controlled Trial with 412 Randomized Patients
J Falutz1, S Allas2, K Blot2, D Kotler3, M Somero4, D Berger5, S Brown6, G Richmond7, J Fessel8, and Steven Grinspoon*9
1Montreal Gen Hosp, McGill Univ Hlth Ctr, Canada; 2Theratechnologies, Inc, Montreal, Canada; 3St Lukes Roosevelt Hosp Ctr, Columbia Univ Coll of Physicians and Surgeons, New York, NY, US; 4Palm Springs, CA, US; 5Northstar Hlth Care, Chicago, IL, US; 6AIDS Res Alliance, West Hollywood, CA, US; 7Fort Lauderdale, FL, US; 8Kaiser Fndn Res Inst, San Francisco, CA, US; and 9Massachusetts Gen Hosp, Boston, US
Background: A significant proportion of HIV patients treated with ART develop increased visceral adipose tissue (VAT), a known cardiovascular risk factor. No medical treatment is approved to reduce VAT. Prior preliminary studies suggest that treatment with GRF (GHRH) decreased visceral fat while preserving subcutaneous fat.
Method: HIV patients (86% male) with evidence of abdominal fat accumulation in the context of treatment for HIV disease (waist circumference [WC] ≥95 cm and waist-to-hip ratio [WHR] ≥0.94 for male, WC≥94 cm and WHR≥0.88 for female), on stable ART, were randomized to TH9507 2 mg (n = 275) or placebo (n = 137) subcutaneously daily for 26 weeks. The primary endpoint was the percentage of change in VAT by abdominal CT. Secondary endpoints included triglyceride level, cholesterol-to-HDL ratio, and IGF-I. Safety endpoints included glucose and insulin. The study was analyzed by intent-to-treat analysis, and ANCOVA and had 90% power to detect an 8% reduction in VAT between TH9507 and placebo. Final data are presented.
Results: Baseline age was 48±7 years, WHR 1.1±0.1, and WC 104±10 cm (mean±SD). Study population included 19% with T2DM or glucose intolerance. Study parameters were similar at baseline between groups. In all, 80% of subjects completed 26 weeks. At week 26, VAT decreased significantly (-15.2±20.8 vs +5.0±23.4%, p <0.001, TH9507 vs placebo. Trunk fat by DEXA also decreased (-1.0±1.9 vs +0.4±1.6 kg, p <0.001), whereas lesser changes were observed in abdominal SAT (+0.4±15.8 vs +1.8±14.5%, TH9507 vs placebo, p = 0.05) and limb fat (-0.0±0.8 vs +0.2±1.0 kg, TH9507 vs placebo, p = 0.01). The lipid profile improved with significant reduction in triglycerides (-0.6±1.7 vs +0.1±1.3 mmol/L, p <0.001, TH9507 vs placebo) and in cholesterol to HDL ratio (-0.3±1.0 vs +0.2±1.0, p <0.001, TH9507 vs placebo). Mean IGF-I increased within the physiological range (+80% vs -5%, TH9507 vs placebo, p <0.001). Overall, treatment was well tolerated. The number of patients with adverse events was not different between the groups (p = 0.12). Adverse events in >10% of subjects were headache (16 vs 18%, p = 0.78) and arthralgia (13 vs 10%, p = 0.43). No significant changes were seen in fasting and 2-hour glucose and insulin at week 26.
Conclusions: These data indicate that daily administration of 2 mg TH9507 for 26 weeks preferentially decreased VAT and improved lipid profile. TH9507 was well tolerated and may represent a novel treatment strategy for HIV patients with central fat accumulation, including those with impaired glucose homeostasis.
|
| |
|
 |
 |
|
|