 |
 |
 |
| |
Bioequivalence of Pilot Tablet Formulations of Ritonavir (RTV) to the Marketed Soft gel Capsule (SGC) at a Dose of 100 mg
|
| |
| |
Reported by Jules Levin
CROI, Feb 25-28, 2007, Los Angeles
George Hanna from Abbott reported this presentation.
Study Conclusions:
-- Ritonavir prototype tablet formulations A and B met bioequivalence criteria with respect to RTV Cmax, AUCt and AUC.
-- Ritonavir prototype tablet formulation C resulted in a statistically significantly greater RTV Cmax, AUCt and AUC compared to the SGC.
-- All formulations were generally safe and well tolerated at a single dose of 100 mg used in this study.
NEXT STEPS
There are several ongoing studies to guide the selection of a formulation suitable for registration:
-- Stability evaluations under a variety of temperature, humidity and packaging material conditions
-- Bioavailability assessment at the registered dose of 600 mg
Required activities after selection of a formulation for registration:
-- Production scale manufacturing process development followed by manufacture of registration batches
-- Once these registration batches are complete they will need to conduct a pivotal bioequivalence study using registration batches
-- as well as stability testing using registration batches for 12 months duration before they are able to file
Note from Jules Levin: it appears this process could take 2 years before filing for approval.
Background
Ritonavir (RTV) is an HIV-1 protease inhibitor indicated for the treatment of HIV-1 infection in combination with other antiretroviral agents.
-- Indication is based on RTV use as an antiretroviral dosed 600 mg BID.
-- RTV is widely used as a pharmacokinetic enhancer at low doses (typically 100 mg QD to 200 mg BID).
Historically, solid formulations of RTV showed poor bioavailability
-- RTV is a low solubility, low permeability drug (BCS Class 4)
-- Unformulated RTV solids have bioavailability < 5%
-- Two liquid based formulations currently available
RTV 100-mg soft gelatin capsules (SGC)
RTV 80 mg/mL oral solution
Introduction
Solubility of RTV is critical for bioavailability
Emergence of less soluble crystal form of RTV in 1998 had a significant impact on RTV concentration and storage temperatures of liquid formulations:
-- More concentrated formulations are prone to RTV crystallization, particularly at low temperatures
-- RTV in liquid formulations is prone to degradation, particularly at high temperatures
Storage recommendations balance requirements for RTV solubility with need to minimize RTV degradation for each of the current liquid-based formulations:
-- RTV solution (80 mg/mL in ethanol-based solution) stored at room temperature (20 -25 C) with shelf life of 6 months
-- RTV SGC (~100 mg/gm in oleic acid-based solution) stored refrigerated until dispensed, then may be stored at room temperature for up to 1 month
Application of Melt Extrusion Technology to Ritonavir
A 100-mg tablet formulation of RTV using melt-extrusion (MeltrexTM) technology that eliminates the SGCÕs requirement for refrigeration is being developed.
Previous efforts at developing a tablet formulation using other technologies produced unacceptably low bioavailability.
A tablet form of lopinavir/ritonavir (LPV/r) 200/50 mg was recently introduced using melt-extrusion technology.
-- No requirement for refrigerated storage
-- Significantly reduced food effects compared to the SGC1
The amount of RTV for the RTV tablet (100 mg) is double the amount in the LPV/r tablet, requiring additional formulation work to produce a tablet of acceptable size.
Application of Melt Extrusion Technology to Ritonavir
MeltrexTM technology: Solid dispersion formulation
-- Significantly improves the bioavailability of poorly soluble compounds like RTV with solvent-free technology. Unlike a traditional tablet, which generally contains small drug crystals mixed with excipients, a solid dispersion formulation has drugs which remain in a non-crystalline state which is in a hydrophilic polymer which allows the drug remains in a state which allows for an extended shelf life (and for LPV/r this was 2 years).
Objectives of Tablet Formulation Program
-- Eliminate need for refrigeration
-- Bioequivalence to the SGC formulation
-- Allow for chemical and physical stability over a range of temperature and humidity conditions
-- Acceptable tablet size
Three pilot formulations developed (Form A, B and C)
--each of these pilot formulations are of a size smaller than the ritanovir soft gel capsule
Study objective:
- To compare the single-dose bioavailability of three pilot RTV tablets with that of the marketed reference SGC at a dose of 100 mg under non-fasting conditions.
METHODS
This was a Phase I, single-dose, four-period, randomized, partial-crossover study in HIV-negative healthy adult subjects (N=32). All subjects received the reference soft gel capsule, not all subjects all 3 of the pilot formulations.
-- A single dose of RTV (100 mg) was administered 30 minutes after a moderate-fat meal with a washout interval of at least 7 days between periods.
-- Serial blood samples were collected for RTV concentration for 36 hours after dosing.
Preliminary Analysis on the Final Data Presented Today
Following PK parameters were determined on Day 1 of each period: Tmax, Cmax, AUCt, AUC, t1/2, CL/F.
-- Statistical analysis was performed for Tmax, b and the logarithms of Cmax, AUCt and AUC , and the bioavailability was assessed by the two one-sided test procedure via 90% confidence interval (CI).
Safety and tolerability were assessed throughout the study.
DEMOGRAPHIC SUMMARY
Study was performed in Germany.
84% men, 100% white. Average age 40 yrs. Mean weight & height: 77 kg, 197 cm.

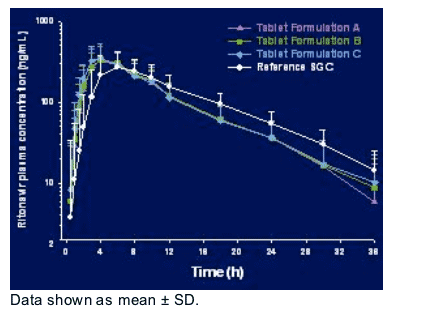
Ritonavir Plasma Concentrations-Time Profile
As you can see in this figure the tablet formulations tended to have a more rapid absorption producing a slightly higher Cmax compared to the reference soft-gel capsule. Nevertheless, the exposures were in general were very similar to that seen with the reference soft-gel capsule at a dose of 100 mg.
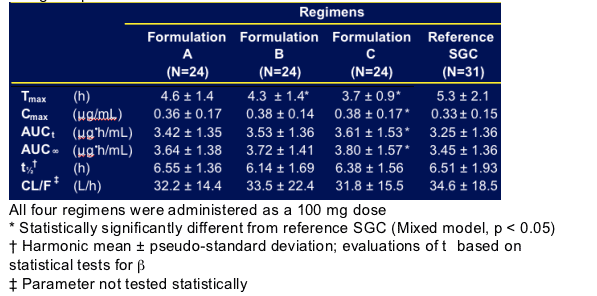
Ritonavir Pharmacokinetic Parameters, Mean ± SD
For formulation A & B, Cmax, AUC, half-life, and oral clearance were not statistically significantly different from the reference soft-gel capsule. However, for formulation C, Cmax and AUC were statistically significantly greater than the soft-gel capsule.
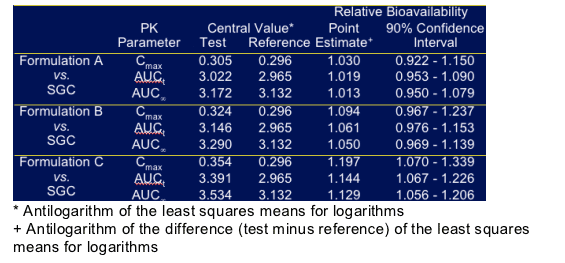
Ritonavir Bioequivalence Assessment
For formulation A & B, the point estimate of the ratio for Cmax & AUC between the formulation and the soft-gel capsule was very close to 1 with a 90% confidence interval well within the 0.8 to 1.25 interval required to demonstrate bioequivalence. In contrast for formulation C the point estimate for Cmax was 20% higher and for AUC about 13-14% greater.
Safety Assessment
All regimens were generally safe and well tolerated.
-- No subjects were discontinued from the study due to AEs.
All adverse events were mild or moderate in severity. The majority of adverse events were judged to be not related to study drug.
There was only one treatment-emergent AE judged as probably related to the study drug that was mild in severity.
No adverse event was reported by two or more subjects.
|
| |
|
 |
 |
|
|