 |
 |
 |
| |
Single Agent Therapy with Lopinavir/ritonavir Controls HIV-1 Replication in the Central Nervous System
|
| |
| |
Reported by Jules Levin
CROI, Feb 25-28, 2007, Los Angeles
RF Yeh1,2, S Letendre3, IS Novak4, BA Lipman5, A Hermes5, C Mayberry1, B Miguel1, J Nemecek6, M Norton5, JC Gathe Jr.11Therapeutic Concepts, Houston, TX; 2University of Houston, Houston, TX; 3University of California, San Diego, CA; 4Park Plaza Hospital, Houston, TX5Abbott Laboratories, Abbott Park, IL; 6Donald R. Watkins Foundation, Houston, TX
Background
Lopinavir/ritonavir (LPV/r) as single-agent therapy has exhibited virologic suppression comparable to combination HAART when utilized in a variety of treatment strategies. 1-6
Inability to control HIV replication in sanctuary sites such as the central nervous system (CNS) may limit success of antiretroviral therapy. 7
LPV/r has demonstrated control of HIV replication when used in combination with other antiretroviral therapy (ART). Although LPV concentrations in CSF are lower than plasma, studies have shown concentrations exceed levels that suppress wild-type HIV replication. 8 9,10
Previous studies not detecting LPV in the cerebral spinal fluid (CSF) have been limited by insufficient assay sensitivity.8
Previous data describing CNS antiretroviral activity of LPV/r when used as a sole agent was limited by a short duration and was prior to full suppression. 11
Methods
IMANI-2 is an ongoing, prospective, open-label investigation of LPV/r in 40 antiretroviral naive HIV-1 infected subjects. LPV/r is administered as a single-agent, 400/100 mg twice daily.
Substudy was approved by the Institutional Review Board and all participating subjects provided written, informed consent.
Inclusion criteria:
All subjects enrolled in IMANI-2 who had reached at least week 24 with 2 most recent plasma HIV-1 RNA < 75 copies (Bayer Versant HIV RNA 3.0, bDNA) by September 15, 2006, were approached for enrollment in this sub-study. Plasma HIV-RNA were performed by LabCorp¬.
Exclusion criteria:
Contraindication to lumbar puncture (LP) (e.g. coagulopathy).
LPs were performed by a board certified neurologist using standard technique.
LPs were performed at median week 38 (range 24-48).
CSF samples from 3 subjects for HIV-RNA were obtained 0-6 hours after dosing and the remaining samples were obtained 6-12 hours after dosing. Plasma was also sampled at the time of LP. The median time between CSF and plasma sampling was 37 minutes (range -31-112).
As there is no validated commercially available bDNAassay for CSF, HIV RNA in CSF was measured via RT-PCR (Roche Amplicor ultrasensitive version 1.5) with limits of detection at < 50 copies/ml.
LPV was measured by liquid chromatography mass spectrometry (LC-MS) in CSF and high performance liquid chromatography (HPLC) in plasma (lower limit of quantification (LLOQ) of 100 ng/mL and an inter-assay variability of <7.5%.
LC-MS assay was modified from previously published version.8LPV and internal standard (13C-amprenavir) were isolated from matrix by solvent extraction with methy-tert-butylether (MTBE), resulting in a LLOQ of 0.5 ng/mL with variability <10%.
LPV concentrations in CSF were compared to LPVÕs50% inhibitory concentration (IC50) derived from a panel of 2381 wild-type HIV-1 isolates by the Monogram Biosciences PhenoSense assay [median 3.0 nmol/L (1.9 ng/mL)].12
IMANI-2 CSF Sub-study Subject Disposition
22 subjects met inclusion criteria. 12 subjects received LPs: 1 specimen could not be evaluated due to traumatic LP. 10 subjects did not receive LP: 7 subjects declined to participate in LP substudy; 3 subjects LP contraindicated per Neurology.
RESULTS
Of the twelve CSF samples obtained, 11 were evaluable.
One CSF sample was excluded because the LP was traumatic (RBC 125, WBC 3, 128 HIV-RNA copies/ml.
Included subjects represented diverse racial/ethnic background, age, and gender. (Table 1)
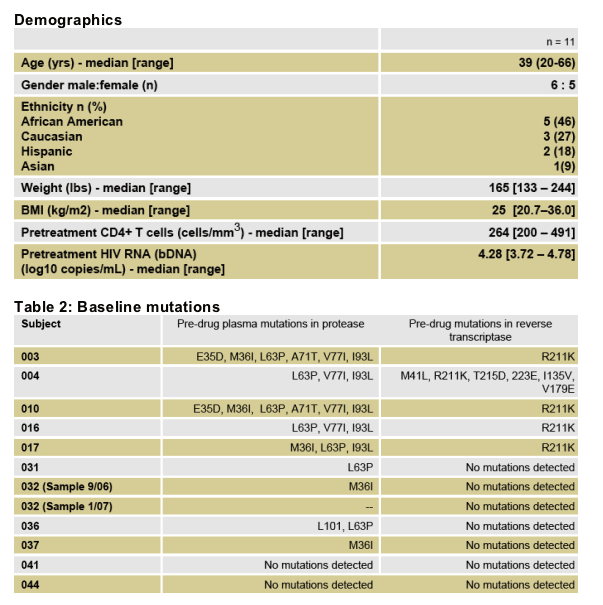
Median LPV/r exposure at the time of LP was 38 weeks (range 24-48)
Median blood CD4+ cells at LP were 471 cells/mm3 (range 265-769)
All subjects were asymptomatic at the time of LP (Table 3)
Table 3: Plasma viral load, CD4+ and week of LP
All patients had undetectable plasma copies/ml (<75). CSF HIV RNA was <50 in all subjects except 2: 251 copies in 1 patient, 747 copies in 2nd patient).
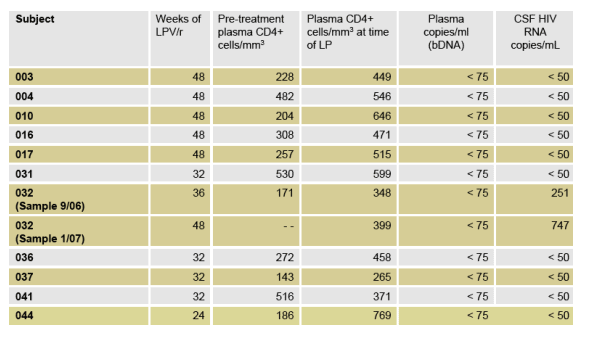
11/11 subjects had quantifiable LPV plasma concentrations.
Median plasma LPV concentration was 9679 ng/mL (range 4887-17003).
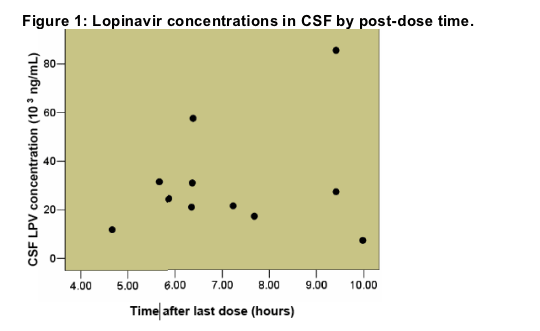
Median CSF LPV concentration was 24.3 ng/mL, mean 30.4 ng/mL (range 7.1 Ð85.3).
The median LPV/IC50 ratio is 12.8, mean 16.0 (range of 3.7 Ð44.9).
All subjects had LPV concentrations of at least 3-fold above the median IC50 for wild-type HIV-1.
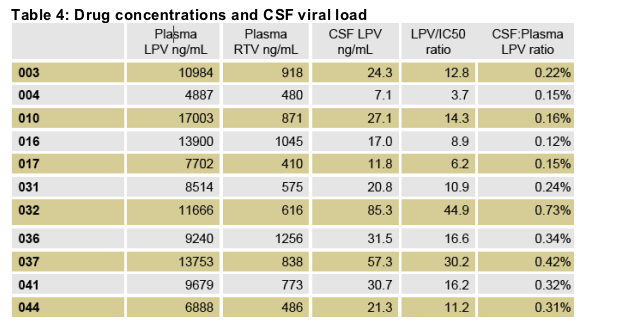
LPV median post-dose CSF sampling interval was 6.38 hours (range 4.67-9.98)
Post-dose plasma sampling interval was 6.0 hours (range 4.08-8.77).
The median CSF-plasma ratio was 0.24% (range 0.12-0.73).
The relationship between time after last dose and CSF concentration is displayed in (Figure 1).
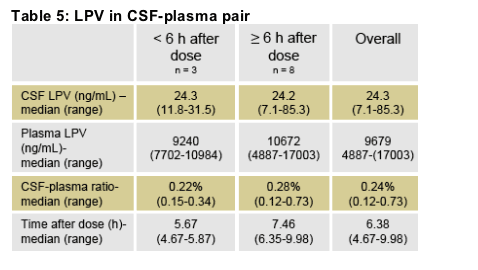
Subject 32:
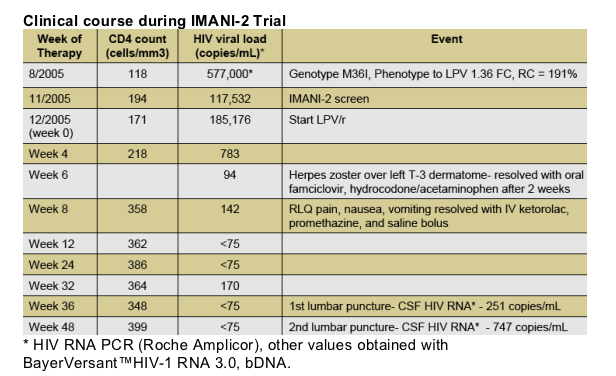
The subject had quantifiable HIV RNA in 2 CSF specimens (251 and 747 copies/ml)). On first LP, LPV CSF concentrations were not obtained. Second LP found a LPVCSF concentration of 85.0 ng/mL. This value exceeded 3 times the median LPV CSF concentration for the cohort. This value was the highest CSF LPV concentration found in our trial. Genotypic resistance testing was performed on HIV derived from CSF revealing no primary mutations in protease.
Detailed Clinical Summary of Subject 032
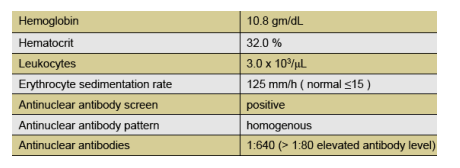
Subject 032 is a 36 year old Asian male born in Vietnam, residing in the U.S. since 1989, who was referred from a rheumatology clinic after evaluation in August 2005 for left-sided headache, decreased vision, pruritic maculopapular rash, joint pain, and right lower quadrant pain. He had unremarkable social history, no known HIV risk factors, was not receiving any medications and had no known allergies. Laboratory results were as follows:
He was diagnosed with optic neuritis and suspected systemic lupus erythematosus, treated with intravenous methylprednisolone and prednisone tapered over 3 weeks. When he did not respond, he was referred to our clinic where he was found to have:
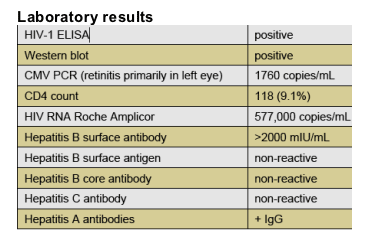
Other infectious disease work-up was negative. CMV retinitis was treated with intravenous ganciclovir for 4 weeks and maintenance therapy with oral valganciclovir prior to screening for IMANI-2.
DISCUSSION
This study demonstrated that single therapy with LPV/r suppressed CSF viral loads in all but one subject. Concentrations of LPV in the CSF while significantly lower compared to blood plasma, are likely adequate for suppression given the relatively protein free environment ofCSF. In addition all CSF LPV concentrations did exceed the Monogram Biosciences median IC50 and 99thpercentile IC50 [8.1 nmol/L (5.1 ng/mL)] for wild-type HIV-1.
1.Persistent HIV replication can occur in the plasma during treatment with boosted protease inhibitors in the absence of identifiable resistance.13The reasons for this are not completely understood but this phenomenon might also occur in other compartments, such as the CNS. Detectable HIV RNA levels in CSF have been previously reported during otherwise successful therapy with triple agent HAART.14,15,
2.HIV replication in CSF could persist if LPV concentrations weresub-therapeutic. The LPV concentration in the CSF of this individual, however, was the highest in the group and exceeded the Monogram Biosciences median IC50 and 99thpercentile IC50 by approximately 45-and 17-fold, respectively.
3.HIV with reduced susceptibility to LPV could continue to replicate in the presence of higher levels of LPV. However, genotype resistance testing of HIV derived from the CSFof this individual demonstrated the absence of primary protease resistance mutations.
4.Protease inhibitors inhibit production of infectious virionsbut not transcription of viral DNA or translation of viral polyproteins. In the absence of reverse transcriptase inhibition, viral RNA and polyproteinscan accumulate in infected cells. This non-infectious viral RNA can be measured by RT-PCR assays, especially if the infected cells lyseduring processing. Leukocytes were present in both CSF specimens from this individual and, according to the clinical lab, all leukocytes lysedin the second specimen before they could be counted. Since this subject had therapeutic levels of LPV in CSF and no evidence of reduced susceptibility, the production of intact virionsis less likely. Instead, the measured RNA may have reflected release of intracellular HIV RNA from lysedlymphocytes.
5.If the HIV RNA in this CSF specimen derived from lysis of trafficking replication competent lymphocytes, greater trafficking should lead to higher HIV RNA levels. We hypothesized that this individual might have high MCP-1/CCL2 levels in CSF because this potent chemokine induces trafficking of monocytes and lymphocytes into the CNS16,17. The MCP-1/CCL2 concentration in this specimen was 934 pg/mL, a level that is at the ~90th percentile of an independent cohort of 119 HAART-treated individuals and is ~4 times more likely to come from an individual who has an A-to-G polymorphism in the promoter region of the gene encoding MCP-1/CCL218,19. Notably, MCP-1 has also been implicated in the neurologiccomplications of HIV20and systemic lupus erythematosus216.Another possible explanation for the finding depends on the differences between CSF and brain tissue. Since CSF provides an imperfect window into brain events, HIV and LPV characteristics in CSF may not accurately reflect those in the brain. For example, the persistent HIV RNA in CSF could derive from compartmentalized replication in microgliaor brain macrophages22,23, which is more common in those with CD4 nadirs under 200/_L, and sub-therapeutic LPV concentrations in brain tissue despite having therapeutic concentrations in CSF. At present, this theory cannot be proven without obtaining brain tissue.
CONCLUSION
This study is the first to examine CSF viral load in a diverse cohort of na•ve subjects treated with LPV/r single-agent therapy for at least 24 weeks. Subjects were sampled throughout the dosing interval and 10 of 11 achieved suppression of CSF viral load <50 copies/mL.
The LPV CSF median concentration of 24.3 ng/mL (range 7.1-85.3) in single agent therapy was above the previously reported median of 17.0.7The median LPV/IC50 ratio in this study was 12.8 (mean 16.0) with a range of 3.7 to 44.9. All individual subject LPV concentrations exceeded the reference population median IC50 by at least 3-fold, and the mean CSF LPV concentration exceeded the reference population median IC50 by 16-fold. Therefore LPV/r delivers adequate LPV concentrations that reliably exceed the reference population median IC50 for wild-type virus.
In 10 of 11 subjects LPV/r given as single-agent therapy effectively controlled viral replication in the CSF compartment.
The implications of potential CNS viral replication during ARV in the absence of resistance, whether using single agent therapy or triple agent HAART, warrants further study.
|
| |
|
 |
 |
|
|