 |
 |
 |
| |
In Vitro Resistance Profile of HIV-1 Mutants Selected by the HIV-1 Integrase Inhibitor, GS-9137 (JTK-303)
|
| |
| |
Reported by Jules Levin
CROI, Feb 2007, Los Angeles
G Jones,* RM Ledford,* F Yu, X Chen, MD Miller, M Tsiang, and DJ McColl
Gilead Sciences, Inc, Foster City, CA, USA
AUTHOR CONCLUSIONS
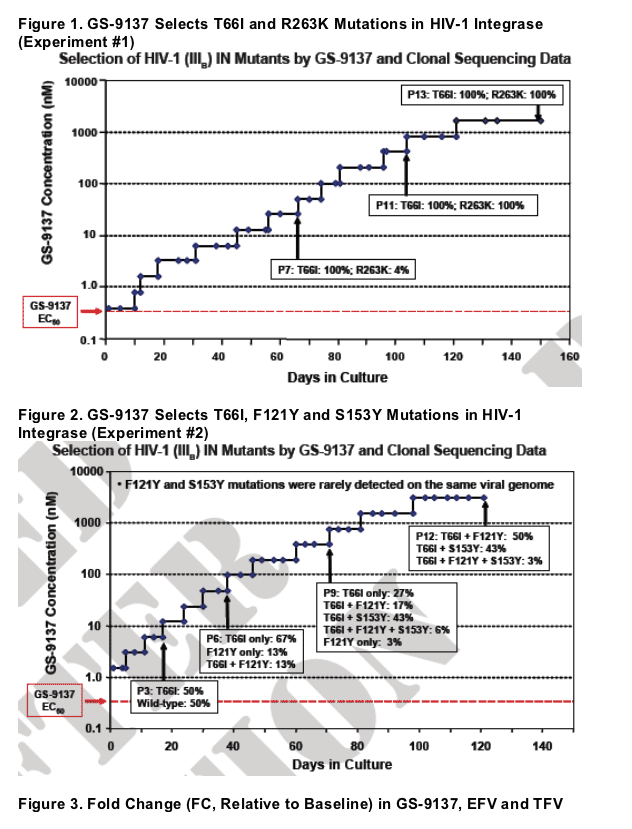
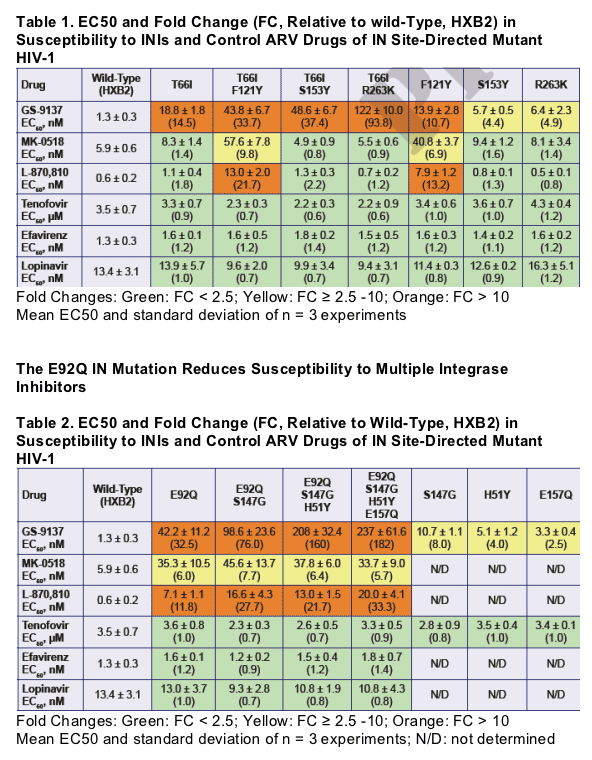
GS-9137 can select two primary resistance patterns involving either T66I or E92Q mutations in HIV-1 integrase
- The T66I mutation alone causes reduced susceptibility to GS-9137
(about 15-fold) but does not reduce susceptibility to MK-0518
- T66I has been observed to be selected by other INIs4
- The E92Q mutation causes reduced susceptibility to multiple INIs, including GS-9137 (≥ 30 fold reduced) and MK-0518
(about 6-fold reduced)
Additional secondary IN mutations selected by GS-9137 include
H51Y, F121Y, S147G, S153Y, E157Q and R263K
- These secondary mutations cause low-level reduction in GS-9137 susceptibility and further reduce GS-9137 susceptibility when added to the T66I or E92Q mutations
- The F121Y and S153Y IN mutations have been observed to be selected by other INIs4,5
- The F121Y and S153Y mutations were rarely found on the same viral genome
- Viruses containing the F121Y IN mutation have reduced susceptibility to MK-0518 (about 7-fold)
- The R263K mutation is novel among the mutations selected by GS-9137 in being located in the C-terminal domain of IN, whereas the other mutations selected by GS-9137 cluster in the catalytic core domain HIV-1 IN mutations selected by GS-9137 have no effect on susceptibility to other ARV drugs, including NRTIs, NNRTIs and PIs
Background
Human immunodeficiency virus type 1 (HIV) encodes three enzymes essential for viral replication:
-- reverse transcriptase (RT), protease (PR) and integrase (IN)
Currently approved antiretroviral (ARV) drugs, including NRTIs, NNRTIs and PIs, inhibit the enzymatic activities of RT and PR
The evolution of viral drug resistance is a significant problem for the management of HIV-1 infected patients; thus development of new ARV drug classes is essential
GS-9137 is an inhibitor of both HIV-1 and HIV-2 IN, with an EC50 in the low nanomolar (nM) range1
GS-9137 is also active against HIV-1 isolates resistant to all currently approved ARV drug classes2
GS-9137 is undergoing Phase 2 dose-ranging studies as a once-daily, ritonavir-boosted therapy in antiretroviral-experienced HIV-1 infected patients
The potential of GS-9137 to select HIV-1 variants with reduced susceptibility was analyzed by performing resistance selection experiments in vitro
Previous experiments in vitro with GS-9137 have demonstrated the selection in HIV-1 (IIIB) of an E92Q IN mutation as a primary GS-9137 resistance mutation1
Two additional independent selection experiments were performed in vitro with GS-9137 and HIV-1 (IIIB strain) to identify additional IN mutations that can be selected by GS-9137
OBJECTIVES
Characterize the susceptibility to GS-9137 and control ARV drugs, including integrase inhibitors (INI), of HIV-1 site-directed mutants containing the E92Q and associated IN mutations (S147G, H51Y, E157Q) selected by GS-9137 in vitro
Characterize the genotypic changes and associated ARV phenotypes of HIV-1 IIIB variants selected by GS-9137 in independent experiments in vitro
RESULTS
References
1. Kodama et al 2006; Poster # H-254, 46th Interscience Conference on Antimicrobial Agents and Chemotherapy.
2. Matsuzaki et al 2006; Poster # 508, 13th Conference on Retroviruses and Opportunistic Infections.
3. Chen et al 2000; PNAS; 97: 8233-8238.
4. Hazuda et al 2000; Science: 287: 646-650.
5. Hazuda et al 2004; PNAS; 101: 11233-11238.
|
| |
|
 |
 |
|
|