 |
 |
 |
| |
A Double-Blind, Parallel, Randomized, Placebo-Controlled, Single and Repeat Dose Escalation Study to Investigate the Safety, Tolerability, and Pharmacokinetics of the HIV Integrase Inhibitor GSK364735 in Healthy Subjects (GRZ105655)
|
| |
| |
Reported by Jules Levin
CROI, Feb 2007, Los Angeles
Sunila Reddy1, Sherene Min1, Julie Borland1, Ivy Song1, Jiang Lin1, Amar Mehta1, Sandra Palleja2, and William Symonds1
1GlaxoSmithKline, Research Triangle Park, NC, US and 2Shionogi USA, Inc, Florham Park, NJ, US
Background: GSK364735 (735) is a potent HIV integrase strand transfer inhibitor. The aim of this study was to determine single and repeat dose safety, tolerability, and pharmacokinetics (PK) of 735 in healthy subjects.
Methods: This was a double blind, randomized, parallel, placebo-controlled, Phase I study. In Part A, 3 alternating cohorts of 10 subjects (8 active/2 placebo) received single doses of 50-400mg fasting; 200mg and 400mg + food, 735 50mg + RTV 100mg and 735 200mg + Maalox ES 30cc. In Part B, 5 cohorts received repeat-doses of 100-600mg daily with food for 7 days. CYP450 probes were administered before and after repeat doses of 735 200mg q12h. Safety was assessed throughout the study. Serial blood samples were analyzed for 735 plasma concentrations using a validated LC/MS assay. PK parameters were estimated using non-compartmental methods.
Results: 79 (30 Part A, 49 Part B) subjects were enrolled: The most commonly reported drug-related AEs (n) were headache (5), abdominal discomfort (3), flatulence (2), loose stool (2), lightheadedness (3), sedation (4), and musculoskeletal pain (3). All were mild, except one moderate headache. None were serious or treatment-limiting. No Grade 2 or higher laboratory abnormalities or significant changes in vital signs, telemetry or electrocardiograms were reported. Preliminary PK parameters (geometric mean [CV%]) are presented below.
Table 2. Selected Pharmacokinetic Parameters Following
Single and Repeat Doses (Geometric Mean [CV%])
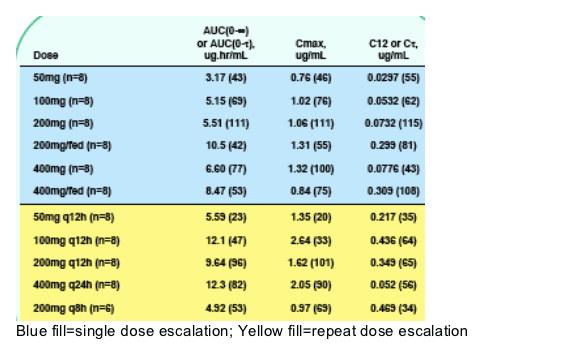
735 exposure increased less than proportionally with dose. Food increased 735 exposure by 30-100%. RTV did not significantly alter the PK of 735. Maalox reduced 735 exposure by about 50%. 735 did not inhibit or induce CYP enzyme
activities except weak inhibition of CYP1A2 which was not considered clinically significant.
Conclusions: GSK364735 was safe and well-tolerated. 735 exposure exceeded
therapeutic targets (0.062 ug/mL, protein-shift EC90) and is now in Phase II
clinical studies in HIV infected patients.
Clinical Safety
-- No severe or serious clinical AEs have been reported. No subjects discontinued 735 due to an adverse event.
-- The most commonly reported AEs with 735 SD and RD (N=65), excluding the probe cocktail, were: application site erythema at ECG leads (7 subjects), headache (6), antecubital erythema (4), & dizziness (3).
-- The most commonly reported drug-related (735) AEs were: headache (5), abdominal discomfort/pain (3), diarrhea (2), flatulence (2) & dizziness (2).
-- All AEs were mild, except moderate headache (1 subject, SD group), viral gastroenteritis (3, RD group), and syncope related to a blood draw (1, RD group).
-- No trends in laboratory abnormalities were apparent. No treatmentemergent grade 2 or higher labs were reported, except elevated glucose (2).
-- No clinically significant changes were reported on telemetry or VS.
-- No clinically significant changes were reported on serial ECGs, including
no QTcB or QTcF prolongation (QTc = 500msec or delta baseline =60msec).
Predicted Therapeutic Target
-- The antiviral activity of integrase inhibitors is likely associated with Cmin
-- EC50 value from PBMC assay = 1.2nM (geometric mean)
-- Predicted Therapeutic target for Cmin: Protein-adjusted EC90 (PA-EC90)
based on protein shift: 168 nM (=0.062 ug/mL)
PK Summary
Single Dose
-- 735 was readily absorbed and plasma concentration declined in a biexponential manner after reaching the maximum levels between 0.75-4.0h
post dose (fasted conditions). Terminal t1/2 was estimated to be 3-7h.
-- 735 exposure increased less than proportionally with dose and appeared to reach a plateau at doses above 100mg.
-- Co-administration with a meal increased 735 exposure by 24-92%, prolonged tmax, and reduced inter-subject variability at the 200mg dose.
-- Co-administration with Maalox ES under fasted conditions reduced 735 exposure by 44% for AUC and 55% for C12, on average.
-- A single dose RTV 100mg did not significantly alter C12 of 735.
Repeat Dose
-- Steady-state was achieved after 6-7 days of dosing. Average accumulation ratio after twice-a-day dosing was 1.1-1.6 for AUC, 1.1-1.8 for Cmax, and 1.5- 2.8 for C_. 735 showed time invariant PK.
-- Probe study: there was no significant induction or inhibition of enzyme activity of CYP3A4, 2C19, 2C9, and 2D6 by 735. A weak inhibition (20%) of CYP1A2 was observed.
Conclusions
735 was safe and well-tolerated as single and repeat doses.
-- A single dose of ritonavir (100mg) did not increase the exposure of 735, indicating that CYP3A4 is unlikely a major route of elimination for this compound.
-- Maalox significantly reduced 735 exposure and co-administration with metal-cation containing products/dietary supplements will be avoided in future 735 clinical studies.
-- 735 did not inhibit or induce the enzyme activities of CYP3A, 2C9, 2C19, and 2D6 except for weak inhibition of CYP1A2 which was not considered clinically significant. These results provide guidance for future drug interaction studies of 735.
-- 735 exposure exceeded therapeutic target and is now in Phase II clinical studies in HIV infected patients.
-- The pharmacokinetics and food effect of a bioenhanced 735 tablet formulation is currently being investigated in healthy subjects.
|
| |
|
 |
 |
|
|