 |
 |
 |
| |
Efficacy and Safety of Tenofovir DF (TDF)-containing versus
non-TDF-containing Regimens in Black Antiretroviral-Naive Patients
|
| |
| |
Reported by Jules Levin
CROI, Feb 2007, Los Angeles
JE Gallant,1 AL Pozniak,2 S Staszewski,3 E DeJesus,4 SS Chen,5 J Enejosa,5 and AK Cheng5
1Johns Hopkins Univ School of Medicine, Baltimore, MD; 2Chelsea and Westminster Hosp, London, UK;
3University Hospital, J.W. Goethe-Universitat, Frankfurt, Germany; 4Orlando Immunology Center, Orlando, FL; 5Gilead Sciences, Inc., Foster City, CA
Limited data are available on the long-term safety and efficacy of antiretroviral therapy in black HIV-1-infected patients
This study investigated the efficacy and safety of antiretroviral therapy in a sub-population of treatment-naive black patients from 2 randomized, controlled studies
- Study 903: In the overall study population, TDF+3TC+EFV and d4T+3TC+EFV had comparable effi cacy through 144 weeks; TDF arm was associated with better lipid profi les and less lipodystrophy1
- Study 903 enrolled 20% black patients
- Study 934: In the overall study population, the TDF+FTC+EFV arm had
significantly higher proportion of patients achieving HIV-1 RNA < 400 copies/mL compared to AZT/3TC+EFV through 96 weeks; higher limb fat was seen in TDF arm2
- Study 934 enrolled 23% black patients
Study 903 randomized 600 ART-naive patients to TDF/EFV/3TC with d4T placebo or d4T/EFV/3TC with TDF placebo for 144 weeks. Screening GFR >/=60 ml/min. Study 934 randomized 511 ART-naive patients to TDF/FTC/EFV or AZT/3TC+EFV for 144 weeks. Screening GFR >/= 50 ml/min.
METHODS
They evaluated the efficacy and safety of a TDF-containing vs. a thymidine analogcontaining regimen (Control) through 96 weeks in antiretroviral-naive black patients who enrolled in Studies 903 and 934
Main Inclusion Criteria:
- HIV-1 infected, treatment-naive patients
- 18-65 years of age
- Plasma HIV RNA > 5,000 copies/mL (Study 903); >10,000 copies/mL (Study 934)
- No CD4+ cell count criteria
- No significant laboratory or clinical abnormalities
- Estimated GFR by Cockcroft-Gault ≥ 60 ml/min (Study 903); ≥ 50 ml/min (Study 934)
There were no differences between study populations in 903 vs 934 or between the regimens within the study related to gender (58-65% men), mean viral load (4.7 to 5.0 log), and mean CD4 count (198-245).
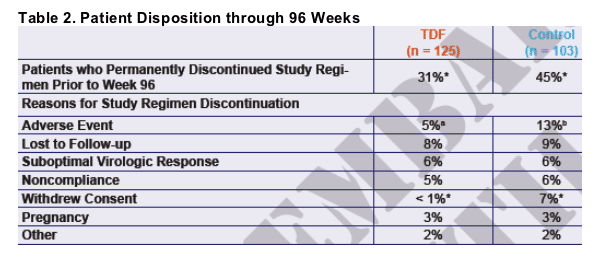
a. Anxiety, bone disorder, cryptococcal meningitis, Kaposi sarcoma, rash
b. Acute renal failure, anemia, dementia, disorientation, dizziness, dry skin, erythema, fatigue, heart failure, lung edema,
lactic acidosis, MAC, pain, periorbital edema, pneumonia, psychotic disorder, rash, vomiting
*p < 0.05 for FisherŐs Exact test comparing the 2 treatment arms

Mean change from baseline to Week 96 in CD4 count was +267 cells/mm3 in the TDF arm versus +233 cells/mm3 in the Control arm (p = 0.08).
Table 4. Renal Parameters
Baseline serum creatinine was 0.90 for TDF & 0.80mg/dL for Control. Change was 0 for both from baseline to week 96. Serum phosphorous was 3.6 & 3.5 mg/dL for TDF & Control at baseline, and change was -0.2 and 0 from baseline to week 96, respectively.
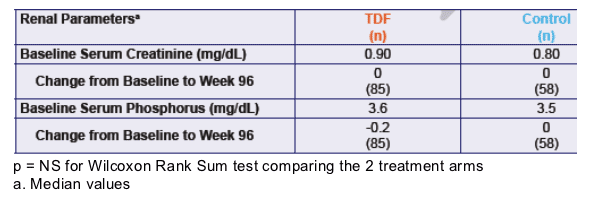
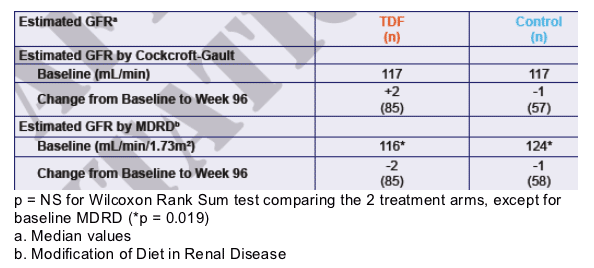
Table 5. Estimated GFR
Estimated GFR by CG at baseline was 117 mL/min for TDF & 117 for Conttol, and change from baseline to week 96 was +2 (n=85) and -1 (n=57), respectively. Estimated GFR by MDRD at baseline was 116 mL/min/1.73m2 for TDF & 124 for Control, and change from baseline to week 96 was -2 (n=85) and -1 (n=58), respectively.

No patient receiving TDF discontinued due to renal adverse events.
1 patient in the Control arm (Study 903) discontinued due to acute renal failure.
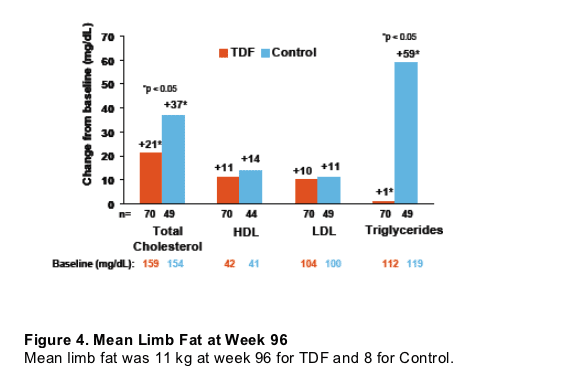
Figure 3. Mean Change from Baseline in Fasting Lipid Parameters through Week 96
Total cholesterol increased by 21 on TDF and by 37 on Control (p<0.05). HDL increased by 11 on TDF & 14 on Control. LDL increased by 10 on TDF & 11 on Control. Triglycerides increased by 1 on TDF & 59 on Control (p<0.05).
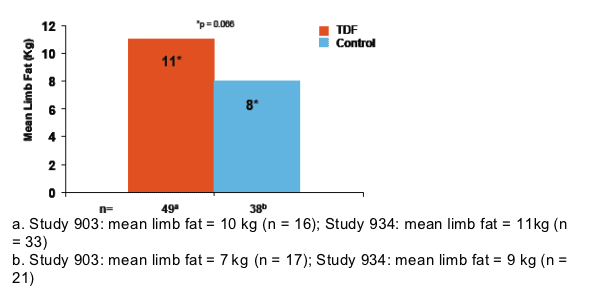
The results of the limb fat analysis are consistent with results from the overall study population
- In Study 903, patients in the TDF arm had higher mean limb fat at Week 96 compared to the d4T arm (8.0 kg [n = 126] vs 5.0 kg [n = 132], p < 0.001)
- In Study 934, patients in the TDF arm had higher mean limb fat at Week 96 compared to the AZT/3TC arm (9.0 kg [n = 144] vs 6.5 kg [n = 136], p < 0.001)
Conclusions
In black antiretroviral-naive patients, TDF-based therapy demonstrated signifi cantly greater virologic suppression compared to thymidine analog-based therapy through 96 weeks.
Renal function in black patients was similar between arms and remained stable through 96 weeks.
Black patients receiving TDF had significantly lower elevations in fasting total cholesterol and triglycerides.
|
| |
|
 |
 |
|
|