 |
 |
 |
| |
Are HIV Docs Managing Lipids Correctly?
|
| |
| |
14th Conference on Retroviruses
February 25-28, 2007, Los Angeles
Side effectology has become a critical, if unwelcome, subspecialty of HIV clinicians. And four lipid studies at the Conference on Retroviruses imply that physicians have become a lot better at fine tuning antiretroviral therapy than at preventing or reversing their side effects. The studies suggest HIV docs do a poor job advising patients on diet and smoking while pursuing antilipid strategies in far fewer patients than need them. Or the studies may mean lots of people with HIV ignore their physicians' good advice on keeping blood fats at bay. Either way, the studies underline a marked need for improvement.
Do people with HIV eat too much saturated fat?
Research points to several factors that may upset normal lipids in people with HIV infection, including an array of antiretrovirals and HIV itself. But what HIV-infected people eat on their way to out-of-line lipid readings has earned less attention, at least until a study of 356 people with HIV and 162 healthy controls by Hester Keogh and coworkers at Massachusetts General Hospital and the National Institutes of Health [1]. Although the non-HIV group downed more saturated fat than the HIV group, people with HIV consumed significantly more calories as saturated fat than the non-HIV group.
Slightly more people in the HIV group took lipid-lowering drugs (12.1% versus 9.9%), a nonsignificant difference (P = 0.18) (but see the next section for a bigger HIV/non-HIV comparison of antilipid therapy). As one would expect, people with HIV had significantly lower CD4 counts (average 444 versus 871 cells, P < 0.0001). The HIV-positive people had been infected for an average 8.5 years, and two thirds were taking a protease inhibitor (PI), which research links to unhealthy lipid levels [2]. Recent work shows, though, that efavirenz contributes to abnormal lipids more than once assumed [3,4].
The HIV and non-HIV groups had similar proportions of men (55.3% HIV, 45.1% non-HIV) and women (44.7% HIV, 54.9% non-HIV), whites (56.3% HIV, 61.1% non-HIV), African Americans (28.4% HIV, 25.3% non-HIV), and Hispanics (9.9% HIV, 6.2% non-HIV). Income quartiles correlated in the HIV and non-HIV groups, and ages averaged 41 to 42 years.
Gauging dietary intake with 4-day food records and diet histories, Keogh found that the "healthy" controls without HIV infection spent more time at the table than infected people, packing in an average 2235 kcal per day versus 2065 in the HIV group, a difference just short of statistical significance (P = 0.08). The non-HIV group gobbled significantly more fat than HIV-infected people (87 versus 79 grams/day, P = 0.02), including more saturated fat (31 versus 27 grams/day, P = 0.004) and monounsaturated fat (33 versus 30 grams/day, P = 0.04). The non-HIV group also swallowed marginally more trans fats (5 versus 3 grams/day, P = 0.09) and ingested significantly more cholesterol (342 versus 294 mg/day, P = 0.004). People with HIV drank significantly more alcohol than controls (7 versus 3 grams/day, P = 0.02).
But because HIV-infected people ingested fewer calories daily than the non-HIV group, they got significantly more calories from saturated fat (P = 0.002) and trans fats (P = 0.02) and marginally more calories from monounsaturated fat (P = 0.11). Furthermore, significantly more HIV-infected people exceeded 2005 USDA daily recommendations for saturated fat (P = 0.003) and cholesterol (P = 0.04), and marginally more people with HIV exceeded guidelines for total fat (P = 0.10).
And high saturated fat quotients had an impact on lipids in people with HIV: Statistical analysis tied saturated fat intake in the HIV group to higher triglycerides (P = 0.005). Male gender also favored higher triglyceride readings (P = 0.001). HIV-infected people had significantly worse average total cholesterol (196 versus 178 mg/dL, adjusted P = 0.003), triglycerides (230 versus 130 mg/dL, adjusted P < 0.0001), "good" high-density lipoprotein cholesterol (41 versus 48 mg/dL, adjusted P < 0.0001), glucose area under the curve (16,980 versus 15,224 mg/dL x 120 min, adjusted P = 0.02), and fasting insulin (13 versus 12 microIU/mL, adjusted P = 0.03). Significantly more people with HIV met criteria for the metabolic syndrome (32.3% versus 22.1%, adjusted P = 0.02).
Non-HIV controls had significantly wider hips (average 107.4 versus 99.8 cm, adjusted P = 0.02), but the HIV group a bigger average waist-to-hip ratio (0.95 versus 0.90, adjusted P < 0.0001). Visceral-to-subcutaneous adipose tissue ratio also proved significantly higher in the HIV group (0.82 versus 0.43, adjusted P = 0.002). As earlier studies found, people with HIV had significantly less arm and leg fat than non-HIV controls (8.3 versus 12.4 kg, adjusted P = 0.0008). The HIV group also had a significantly higher trunk-to-extremity fat ratio (1.5 to 1.0, adjusted P < 0.0001).
Keogh and coworkers concluded that overindulgence in saturated fats contributes to high triglycerides in people with HIV who have other metabolic abnormalities. They urged clinicians to target saturated fats when modifying diets in people with HIV.
Antilipid drugs don't work as well in people with HIV
The high lipids people with HIV suffer because of their diets, their drugs, and their retrovirus don't respond to antilipid therapy as well as high lipids in people without HIV, according to results of a big case-control study involving mostly men at the Kaiser-Permanente health centers in northern California [5]. The worse response, Michael Silverberg and colleagues speculate, may reflect heavier use of certain antilipid drugs that interact less with antiretrovirals and a high rate of PI-plus-nonnucleoside combinations in this population. The Kaiser team figured that poorer control of total cholesterol and triglycerides in their HIV-infected cohort may be clinically meaningful.
Silverberg looked back through records of more than a thousand HIV-infected adults, more than 90% of them men, and compared findings with those from people without an HIV diagnosis matched to the HIV group for age, gender, and year of first dyslipidemia. Kaiser clinicians first diagnosed and treated unhealthy lipids in all study participants after 1995. Kaiser patients qualified for the analysis if they had the following ATP-III-defined lipid elevations:
- Total cholesterol at or above 240 mg/dL (907 with HIV, 5955 without HIV)
- "Bad" low-density lipoprotein (LDL) cholesterol (1) at or above 160 mg/dL, or (2) at or above 130 mg/dL with two or more coronary heart disease risk factors, or (3) at or above 100 mg/dL with coronary heart disease (695 with HIV, 5801 without HIV)
- Triglycerides at or above 500 mg/dL (511 with HIV, 4671 without HIV)
Significantly more people in the non-HIV groups had coronary heart disease or diabetes (34.7% versus 20.6% in the total cholesterol comparison, 48.4% versus 29.5% in the LDL cholesterol comparison, and 49.4% versus 20.6% in the triglyceride comparison). But more people with HIV were coinfected with hepatitis B or C (5.4% versus 1.1%, 7.9% versus 1.4%, and 4.7% versus 1.3% respectively).
From 1996 through 2005, Kaiser clinicians tested significantly more HIV-infected people for total cholesterol and prescribed lipid-cutting drugs significantly more often for the HIV group (P < 0.05 for both comparisons). Over that decade more than 60% of the HIV group versus about 30% of the non-HIV group had a cholesterol test. Heeding drug-interaction guidelines, physicians prescribed lots more pravastatin or atorvastatin for people with HIV than for the non-HIV group (48.3% versus 1.6% in the total cholesterol subgroup, 52.5% versus 1.2% in the LDL cholesterol subgroup, and 36.8% versus 2.5% in the triglyceride group). In contrast, people without HIV got more simvastatin or lovastatin (87.2% versus 33.2%, 93.8% versus 38.7%, and 49.5% versus 21.9% respectively).
Relatively high proportions of people with HIV were taking both a PI and a nonnucleoside--22.8% in the total cholesterol group, 19.4% in the LDL cholesterol group, and 27.8% in the triglyceride group. (The Massachusetts General team did not report how many people combined these two classes [1].) Proportions in the three Kaiser groups taking a PI without a nonnucleoside were 41.6%, 41.2%, and 42.3%. Respective proportions taking no antiretrovirals were 7.4%, 9.8%, and 4.9%.
Through one decade of follow-up, percent changes in total cholesterol, LDL cholesterol, and triglycerides proved significantly more modest in HIV-infected people than in people without an HIV diagnosis (Table 1). HIV-infected people combining a PI with a nonnucleoside had significantly blunted total cholesterol and triglyceride responses when compared with HIV-infected people taking a PI-only regimen (Table 2). Statisticians adjusted both analyses for age, gender, year, baseline lipids, prior coronary heart disease or diabetes, hepatitis B or C, lipid-lowering class, and duration of therapy.
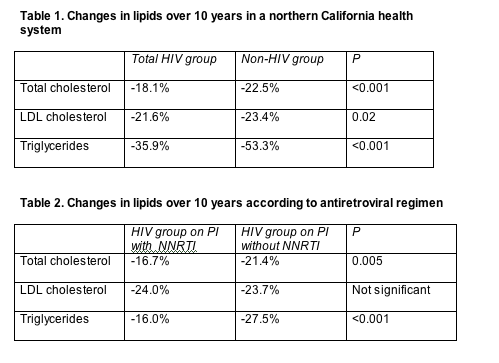
Multivariate analysis determined that people with HIV had a 43% lower chance of reaching ATP-III targets (see above) for total cholesterol, a 19% lower chance of hitting the LDL cholesterol target, and a 61% lower chance of reining in triglycerides (P < 0.05). In more recent years, however, the HIV group had significantly improved odds of attaining lipid targets compared with the non-HIV group (odds ratios 1.13 for total cholesterol, 1.08 for LDL cholesterol, and 1.08 for triglycerides per every later calendar year, P < 0.05 for all). But every extra year of lipid-lowering therapy lowered the chance of controlling lipids in the HIV group versus the non-HIV group (odds ratios 0.85 for total cholesterol, 0.79 for LDL cholesterol, and 0.76 for triglycerides, P < 0.05 for LDL cholesterol and triglycerides).
Looking only at people with HIV infection, Silverberg found that every later calendar year significantly raised the chance of reaching total cholesterol and triglyceride goals (odds ratios of 1.22 and 1.14, P < 0.05 for both), while every extra year of antilipid therapy trimmed the chance of controlling total cholesterol more than 50% (odds ratio 0.44, P < 0.05). In this HIV-only analysis, combining a PI with a nonnucleoside halved the chance of hitting the total cholesterol target (odds ratio 0.52, P < 0.05).
Why did more Kaiser patients with HIV have a better chance of reaching lipid targets in more recent years, yet a worse chance with more years of antilipid therapy? Silverberg proposed via e-mail that lipid-lowering therapies may be improving and that "vigilance to control lipids" may be growing among HIV-infected people and their physicians. An 11-cohort D:A:D analysis described below appeared to support the latter hypothesis.
Why longer lipid-lowering treatment decreased the chance of hitting lipid targets is harder to say. Silverberg pointed out that he limited this analysis to 1 year after starting antilipid drugs, so "this is not the ideal study" to determine how duration of such therapy affects outcomes. "We included [antilipid duration] as a covariate," he explained, "simply to adjust for differences in follow-up time between patients since, for example, some will have a follow-up lipid test close to 1 year, and others may only have a test after 3 months of lipid-lowering therapy."
Are antilipid drugs underused in people with HIV?
D:A:D researchers confirmed Kaiser's finding [5] that antilipid use has climbed over the past several years in the 11 cohorts embraced by this consortium [6]. But Caroline Sabin (Royal Free and University College, London) and D:A:D colleagues found that a concerning majority of HIV-infected people don't start lipid-lowering drugs in the half-year after diagnosis of heart disease or diabetes. And an even bigger proportion continues to smoke.
Sabin and coworkers searched D:A:D cohort records from 1999 through 2006 to identify people with a first diagnosis of diabetes, myocardial infarction, or stroke, or who had an invasive cardiovascular procedure. Then they analyzed use of lipid-lowering drugs and smoking cessation programs before the diagnosis and in the 6 months afterwards. They counted 348 new cardiovascular diagnoses or procedures in people without new diabetes, including 80 (23%) in people already taking antilipid medications.
Of the 268 people not taking lipid lowerers when heart trouble struck, only 121 (45%) started antilipid therapy in the following half year. People with a myocardial infarction or an invasive procedure started lipid-controlling drugs significantly more often than people with stroke (P = 0.007), and people with heart problems in more recent years started antilipid therapy significantly more often than those diagnosed in earlier years (P = 0.007). Men with heart disease were more likely than women to start lipid-lowering agents (P = 0.04), as were people with high total cholesterol or high triglycerides (P = 0.01 for both). But only high total cholesterol independently predicted antilipid therapy after heart trouble. People with a total cholesterol above 6.2 mmol/L (240 mg/dL) had a 2.28 times better chance of getting a lipid-lowering drug (95% confidence interval 1.23 to 4.24, P = 0.009).
Among 149 people who smoked when they had their heart attack, stroke, or invasive procedures, records showed that only 24 (16%) quit cigarettes in the following 6 months. People with a myocardial infarction stopped smoking more than others (P = 0.03), but the overall quitting rate did not improve in more recent years. Only 38 of 184 people (21%) taking a PI when they went to the hospital switched to a non-PI regimen in the half year after their heart problem. Other drugs started within 6 months of cardiovascular trouble included antiplatelet agents in 55%, ACE inhibitors in 22%, and antihypertensives in 40%.
The D:A:D team tallied 626 new diabetes diagnoses since 1999, and 512 of these people (82%) were not taking antilipid drugs at diagnosis. Only 99 of these 512 (19%) started antilipid therapy in the half year after their diabetes diagnosis, and that rate did not improve significantly in more recent years. People with total cholesterol above 6.2 mmol/L were almost three times more likely to start antilipid therapy (odds ration 2.96, 95% confidence interval 1.42 to 6.16, P = 0.004), and people with lipodystrophy proved more than twice as likely to start such treatment after a diabetes diagnosis (odds ratio 2.62, 95% confidence interval 1.74 to 5.52, P = 0.01).
Again, either physicians did not bother to persuade new diabetics to stop smoking, or the attempt failed. Only 10 of 193 people (5%) who smoked when diagnosed with diabetes stopped within 6 months of that diagnosis. And smoking cessation did not gain more traction in recent years. Among 316 people taking a PI when their clinician discovered diabetes, only 86 (27%) jumped to a non-PI regimen within 6 months of their diagnosis. Tiny proportions of people newly diagnosed with diabetes started platelet aggregation inhibitors (1.6%), ACE inhibitors (1.0%), or antihypertensives (1.3%).
The study did not ask why people with a fresh diagnosis of heart disease or diabetes did not turn to antilipid therapy, but Sabin and colleagues speculate that a higher pill burden and potential interactions with antiretrovirals may dissuade some physicians and the people they care for. The researchers note their surprise at the high proportions of people with newly diagnosed heart disease or diabetes who continue PI therapy, even after convincing evidence from this very cohort that longer PI use raises the risk of myocardial infarction [2]. Stopping smoking, they add, "should be emphasized and concerted smoking cessation interventions should be supported."
Is ezetimibe strong enough to take alone?
Ezetimibe, an agent usually combined with statins, lowered dangerous LDL cholesterol all by itself in a 6-week placebo-control study presented by David Wohl (University of North Carolina at Chapel Hill) [7]. But the LDL cholesterol drops recorded with ezetimibe alone suggest its best use remains as a statin adjunct, at least for people who can tolerate statins.
Two earlier trials [8,9], one of them a randomized comparison with fluvastatin [9], found that ezetimibe cuts LDL cholesterol 15% to 20% in 6 months when added to marginally effective statin therapy [8] or given alone [9]. To pursue the potential value of ezetimibe monotherapy in HIV-infected people, Wohl and colleagues randomized 48 people to take 10 mg of the drug daily or placebo for 6 weeks. After 2 weeks without treatment, study participants taking ezetimibe in the first 6 weeks switched to placebo, and vice versa.
Everyone had an LDL cholesterol reading at or above 75 mg/dL and triglycerides under 800 mg/dL while taking a stable antiretroviral regimen. Pretreatment median LDL cholesterol stood at 121 mg/dL and median CD4 count at 555 cells. Twenty-eight people (58%) were taking a PI, all with a ritonavir boost. Three quarters of study participants were men and half were non-Hispanic blacks.
A last-observation-carried-forward analysis determined a median 12% drop in LDL cholesterol with ezetimibe (interquartile range [IQR] -23% to +1%) versus a 3% gain with placebo (IQR -6% to +17%) (P = 0.03). Wohl reported that 35% of study participants saw their LDL cholesterol fall at least 17% after 6 weeks of ezetimibe. High-density lipoprotein cholesterol and triglycerides did not change significantly with either ezetimibe or placebo; neither did CD4 counts.
Three people stopped ezetimibe early because of side effects (elevated liver function test and epigastric pain), while 3 people quit the placebo arm. Two people had liver function test jumps while taking placebo.
D:A:D and EuroSIDA researcher Jens Lundgren voiced his disappointment that ezetimibe monotherapy did not have a bigger impact on LDL cholesterol. While Wohl spoke, Lundgren went online to see if the reported drop in LDL cholesterol improved 10-year cardiovascular risk, and it did not. Still, Wohl and colleagues characterized the LDL cholesterol declines as "clinically meaningful" and suggested ezetimibe merits further study as an option for HIV-infected people who cannot take a statin.
References
1. Keogh H, Joy T, Hadigan C, et al. Increased fat and cholesterol intake and relationship to serum lipid levels among HIV-infected patients in the current era of HAART. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 813.
2. Friis-Moller N, Reiss P, El-Sadr W, et al. Exposure to PI and NNRTI and risk of myocardial infarction: results from the D:A:D study. 13th Conference on Retroviruses and Opportunistic Infections. February 5-8, 2006. Denver. Abstract 144.
3. Haubrich R, Riddler S, DiRienzo G, et al. Metabolic outcomes of ACTG 5142: a prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV-1 infection. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 38.
4. Shikuma CM, Yang Y, Glesby MJ, et al. Metabolic effects of protease inhibitor-sparing antiretroviral regimens given as initial treatment of HIV-1 infection (AIDS Clinical Trials Group Study A5095). J Acquir Immune Defic Syndr. 2007;44. Published online.
5. Silverberg M, Leyden W, Horberg M, et al. Lipid lowering therapy responses in HIV-positive and HIV-negative dyslipidemic patients enrolled in a large integrated healthcare delivery system. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 814.
6. Sabin S, Weber R, Fontas E, et al. Underutilization of recommended interventions for prevention of cardiovascular disease in HIV-infected patients with established cardiovascular disease or diabetes. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 816.
7. Wohl D, Hsue P, Richard S, et al. Ezetimibe's effects on the LDL cholesterol levels of HIV-infected patients receiving HAART. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 39.
8. Negredo E, Molto J, Puig J, et al. Ezetimibe, a promising lipid-lowering agent for the treatment of dyslipidaemia in HIV-infected patients with poor response to statins. AIDS. 2006;20:2159-2164.
9. Coll B, Aragones G, Parra S, et al. Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients. AIDS. 2006;20:1675-1677.
|
| |
|
 |
 |
|
|