 |
 |
 |
| |
TMC114 High Genetic Barrier To Resistance Due to Tight Binding to MDR HIV & Wild-Type
|
| |
| |
Reported by Jules Levin
CROI, Feb 2007, Los Angeles
"Binding kinetics of protease inhibitors to wild-type and multi-drug resistant HIV-1 proteases:
a mechanistic study of the genetic barrier to resistance of darunavir"
De Wit M, Keuleers I, Gustin E, Dierynck I, Hallenberger S, Hertogs K
Tibotec BVBA, Mechelen, Belgium
AUTHOR CONCLUSIONS:
The binding affinity of DRV to WT HIV-1 protease is more than 100-fold higher compared with the other tested PIs, including the structural analogue APV, due to the very high dissociative t1/2 of DRV.
A decrease in binding affinity of all tested PIs on MDR-associated protease variants is mainly caused by an increase in dissociation rate rather than a decrease in association rate. [DRV very high dissociative half-life (t1/2) of DRV to WT protease (>240 hours)].
In contrast to the other PIs, decreases in DRV binding affinity of up to 1000-fold did not compromise the antiviral activity against the corresponding viral strains. This provides a mechanistic background for the higher genetic barrier of DRV to development of resistance compared to APV, ATV, LPV and TPV.
As DRV already starts with a very high affinity (tight binding) to WT HIV-1 protease, it can sustain a substantial decrease in its binding affinity to MDR variants without compromising its antiviral activity. The very high binding affinity of DRV to WT HIV-1 protease necessitates the simultaneous occurrence of multiple mutations before the virus becomes resistant to DRV.
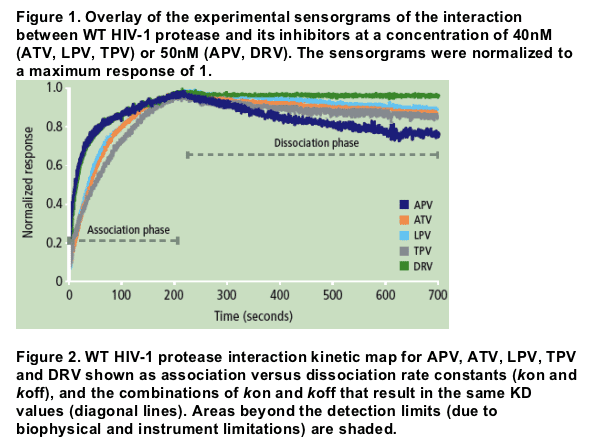
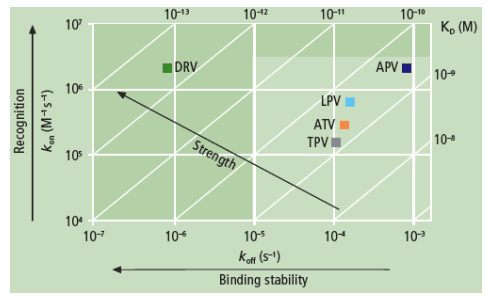
Authors report: "The binding affinity (KD) of DRV for WT protease was more than 100-fold higher compared with APV, ATV, LPV and TPV, mainly due to a very slow dissociation rate (Figure 1). While the association rates of DRV and its structural analogue APV were similar, the more than 100-fold difference in dissociation rate resulted in a much higher binding affinity of DRV to WT protease compared with APV (Figure 2 and Table 2).....
....The high impact of the slow dissociation rate of DRV is clearly illustrated by the very high dissociative half-life (t1/2) of DRV to WT protease (>240 hours) (Table 2). This indicates that the duration of drug action in vivo will be highly extended for DRV in comparison with APV."
Introduction
The high incidence of cross-resistance between HIV-1 protease inhibitors (PIs) limits their sequential use. This necessitates the development of PIs with a high genetic barrier and broad-spectrum activity against PI-resistant HIV, such as tipranavir (TPV) and darunavir (DRV; TMC114).
To investigate the impact of PI resistance-associated mutations (RAMs), a kinetic study was performed with five clinically used PIs: amprenavir (APV), atazanavir (ATV), lopinavir (LPV), TPV and DRV on wildtype (WT) and mutant proteases using surface plasmon resonance (SPR). Five multi-drug resistant (MDR) variant HIV-1 proteases were derived from clinical isolates with a decreased susceptibility to various PIs, and harboring 10-14 RAMs.1
Methods
HIV-1 WT protease and variants were expressed and purified using standard procedures with minor modifications.2,3 All of them included an additional Q7K substitution to prevent autoproteolysis. The protease variants were derived from clinical isolates with a decreased susceptibility to multiple HIV-1 PIs and contained 10-14 PI RAMs based on the IAS-USA October 2005 guidelines,1 as indicated in Table 1.
Interactions between PIs and WT or mutant HIV-1 protease were measured using a Biacore S51 instrument (Biacore AB, Uppsala, Sweden) as previously described.4 HIV-1 protease was immobilized using amine coupling to a CM5 sensor chip (Biacore AB) with an extra cross-linking step to stabilize the surface. The inhibitor studies were performed at 20C with a flow rate of 90_L/min to minimize mass transport effects.
Data were analyzed using a simultaneous non-linear regression analysis (global fitting) with the Biacore S51 or T100 evaluation software (Biacore AB). Individual rate constants kon (association) and koff (dissociation) and a derived affinity constant KD (koff/kon) were determined by kinetic evaluation of the sensorgrams, fitting a 1:1 model if possible, or a heterogeneous ligand model where appropriate. Instrument limitations resulted in detection limits of 104 to 5 x 106 M-1 s-1 for kon and 10-1 to 10-5 s-1 for koff.
Results
The binding affinity (KD) of DRV for WT protease was more than 100-fold higher compared with APV, ATV, LPV and TPV, mainly due to a very slow dissociation rate (Figure 1). While the association rates of DRV and its structural analogue APV were similar, the more than 100-fold difference in dissociation rate resulted in a much higher binding affinity of DRV to WT protease compared with APV (Figure 2 and Table 2).
The high impact of the slow dissociation rate of DRV is clearly illustrated by the very high dissociative half-life (t1/2) of DRV to WT protease (>240 hours) (Table 2). This indicates that the duration of drug action in vivo will be highly extended for DRV in comparison with APV (13 minutes), ATV (1.4 hours), LPV (1.2 hours) and TPV (1.8 hours).
All tested PIs had a lower binding affinity to MDR protease variants containing 10-14 PI RAMs relative to that of WT protease (Figure 3 and Table 2). In general, this decrease was caused by a faster dissociation rate, although for some PI/variant combinations this was also due to a slower association rate (e.g. ATV and LPV on variant D, and APV and TPV on variant E).
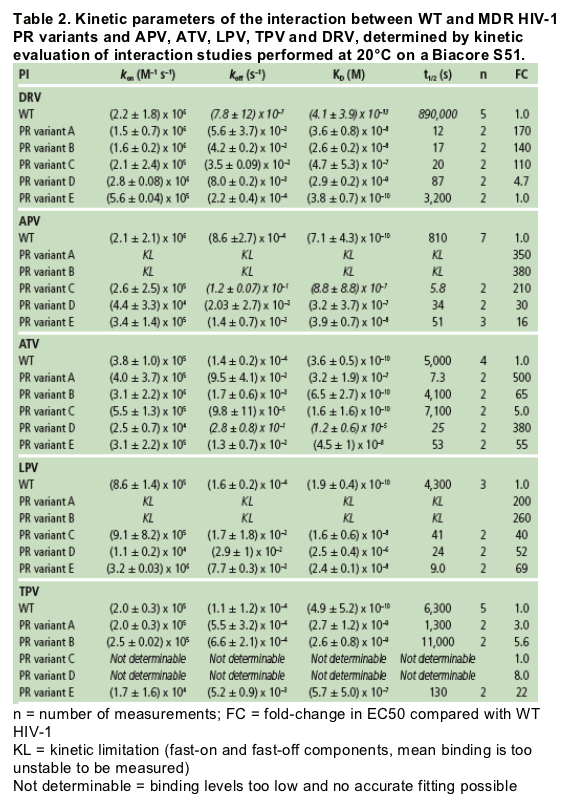
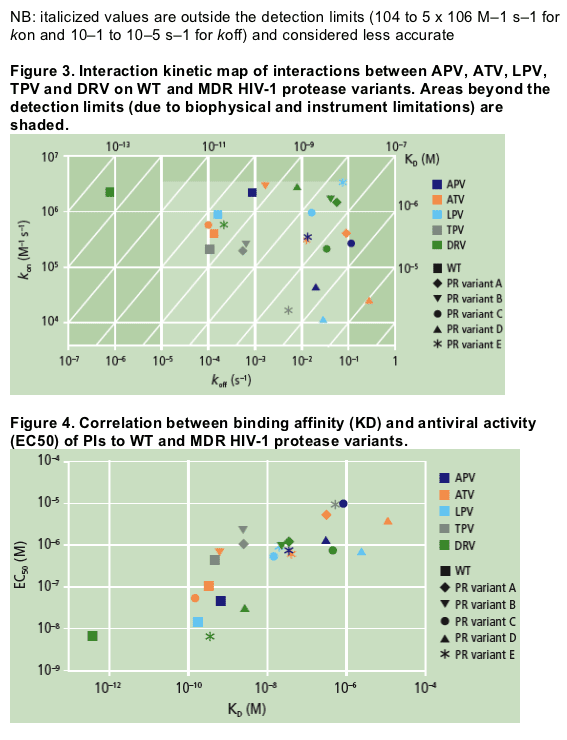
For DRV, decreases in binding affinity of up to a 1000-fold did not affect the antiviral activity against the corresponding viral strain (e.g. variant E), but a further decrease in affinity correlated with a decreased antiviral effect. The antiviral activity of DRV on variant E was as high as on WT protease,
while its binding affinity was about 1000-fold lower. In contrast, APV displayed a 100-fold decrease in binding affinity to variant E, corresponding with lower antiviral activity compared with WT protease (FC in EC50 = 16). This illustrates the higher genetic barrier of DRV compared to APV and the other PIs evaluated in this analysis.
The decreased binding affinity of DRV to MDR variants was proportional to the number of mutations associated with a diminished susceptibility to DRV.5 Variants with 5 (A and B) or 4 (C) of the 11 DRV-RAMs (V11I, V32I, L33F, I47V, I50V, I54M/L, G73S, L76V, I84V and L89V)6 displayed the weakest binding affinity and hence a greater drop in antiviral activity.
The kon of TPV on the MDR variants A, B and E was approximately 10-fold slower than for DRV. For variants C and D, the binding level was too low; together with an association and dissociation rate outside the detection limits, this prevented an accurate determination of the kinetic parameters for TPV (indicated as 'not determinable' in Table 2).
|
| |
|
 |
 |
|
|