 |
 |
 |
| |
Mortality & Causes of Death--and Chances of Avoiding It--With HIV Today
|
| |
| |
14th Conference on Retroviruses
February 25-28, 2007, Los Angeles
Life expectancy with HIV infection surged dramatically in developed countries with the advent of potent antiretroviral combinations. But two big studies showed the substantial (though narrowing) gap that remains between life expectancy of people with and without HIV infection. Other mortality studies presented at the Retrovirus Conference traced shifting causes of death over the past decade in people with HIV and uncovered the importance of CD4 changes over 3 years in predicting death.
Changing AIDS diagnoses and causes of death in France
Tuberculosis replaced Pneumocystis pneumonia, esophageal candidiasis, and Kaposi sarcoma as the first AIDS diagnosis after combination antiretroviral therapy (cART) arrived in France [1]. Risk of death after a first AIDS diagnosis dropped from one era to the next for every diagnosis studied--but the drops proved smallest for progressive multifocal leukoencephalopathy (PML), lymphoma, Mycobacterium avium complex (MAC), and recurrent bacterial infection. AIDS causes of death and (to a lesser extent) non-AIDS causes plummeted in the period of study, so now about half of all deaths can be traced to AIDS and half to non-AIDS diseases.
Sophie Grabar (University of Paris) and colleagues in the French Hospital Database on HIV tracked first AIDS diagnoses and all causes of death in three groups according to when they enrolled in the cohort: 8027 in the pre-cART era of 1993-1995, 3504 in the early cART era of 1998-2000, and 2936 in the late cART era of 2001-2003. Thus the study does not reflect treatment with many of today's leading regimens.
Several things changed over the three periods. Proportions of cohort members infected homosexually or by injecting drugs dropped from era to era, while the proportion of people infected heterosexually rose form 22.3% in pre-cART times, to 40.8% in the early cART era, and to 48.9% in the late cART (P < 0.0001). Changing origins of cohort members probably influenced this shift in infection route, with the percentage of people from sub-Saharan Africa climbing form 2.7% in 1993-1995, to 11.6% in 1998-2000, and to 18.4% in 2001-2003 (P < 0.01). Median age at cohort enrollment rose over the three periods, from 35.3 to 38.2 to 39.8 (P < 0.0001). And the percentage of people with AIDS at enrollment jumped from 25.8% in the earliest period to 38.9% and 37.6% in the two later periods (P < 0.0001).
As the years passed, tuberculosis emerged as the leading first AIDS diagnosis (Table 1), probably partly reflecting the growing influx of Africans into the French cohort. Median CD4 count at first AIDS diagnosis rose for each diagnosis except for Pneumocystis pneumonia (36 cells in 1993-1996 and 33 cells in the two later periods).
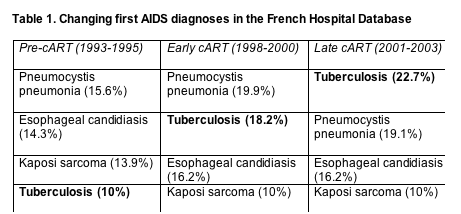
Statistical analysis adjusted for gender, age at AIDS onset, CD4 count, region of origin, ART monotherapy, prophylaxis of opportunists, and other variables that may affect the first AIDS diagnosis determined that the risk of cryptosporidiosis, Kaposi sarcoma, cytomegalovirus infection, and Pneumocystis pneumonia fell more than 80% from the pre-cART to the late cART period. The risk that the first diagnosis would be wasting, toxoplasmosis, herpes, or cryptococcosis fell 70% to 80% across the three eras. The risk that tuberculosis, HIV encephalopathy, or candidiasis would be the first diagnosis declined 60% to 70%. And the risk of a first AIDS diagnosis of recurrent bacterial infection, MAC, lymphoma, or PML fell 50% to 60%.
Three-year risk of death from AIDS-defining causes plunged from 39% in the pre-cART era to 8% in the late cART era. Over the same span, 3-year risk of death from non-AIDS causes slipped from 17% to 9%. As a result, 3-year risk of death from AIDS and non-AIDS causes was equivalent in 2001-2003.
Another French team looked at a later clinical snapshot, comparing 937 HIV-infected people who died in 2005 with 964 who died in 2000 [2]. The proportion of AIDS-related deaths dropped from 47.3% in 2000 to 36% in 2005, but AIDS remained the top killer in this cohort. In the 9% of the population with recent HIV infection, Pneumocystis pneumonia--not TB--persisted as the most frequent diagnosis.
While AIDS-related mortality dropped from 2000 to 2005, numerous non-AIDS causes of death rose: nonhepatitis cancer mortality climbed from 10.5% to 16.4%, hepatitis C infection from 9.3% to 11.4%, heart disease from 7% to 9%, and suicide from 3.9% to 4.9%. The death rate from non-AIDS infections rose from 4.5% in 2000 to 6.8% in 2005, while death rates from hepatitis B infection and other liver ailments stayed flat.
In 2005 AIDS killed 337 people in this cohort, cancer 154, liver disease 136, heart disease 84, and other diseases 226. AIDS and liver disease killed people at median ages of 45 and 46 years, while cancer and cardiovascular disease killed at medians of 50 and 51 years. Overall median CD4 count at death stood at 170 cells, but the median was much lower in people who died of AIDS (42 cells) than in those who died of cancer (212 cells), liver disease (236 cells), or heart disease (344 cells).
Smoking rates were high, even by French standards, in all non-AIDS mortality groups--53% of those who died of heart disease smoked, 62% of those who died from cancer, and 70% of those who died from liver disease--compared with 44% of people who died of AIDS. Nearly half of those who died from liver disease drank "excessive" alcohol, compared with 19% who died of AIDS, 25% who died of cancer, and 21% who died of heart disease.
Death risk and CD4s at 0 and 36 months of therapy
Countless studies show that lower CD4 counts when starting cART boost the risk of progression during treatment. But does the CD4 count 3 years after treatment begins have any prognostic value?
To find out, Jonathan Sterne (University of Bristol) and the ART Cohort Collaboration amassed CD4 and progression data on 18,018 people from 14 cohorts in Europe and North America [3]. Everyone was 15 or older when beginning cART from January 1996 through December 2001, and no one took antiretrovirals before starting a strong regimen. Everyone had at least 36 months of follow-up, so the analysis does not consider people who died within 3 years of starting cART or otherwise dropped out of follow-up.
As one would expect, an unadjusted analysis showed that a lower CD4 count when starting cART correlated with a higher death risk after 36 months of therapy. For example, compared with people starting with 500 CD4s or more, those starting with 0 to 24 cells had almost a doubled risk of death after 36 months (hazard ratio [HR] 1.97, 95% confidence interval [CI] 1.40 to 2.76), and those starting with 50 to 99 cells had a 57% higher risk of death after 36 months (HR 1.57, 95% CI 1.10 to 2.24).
But an analysis adjusted for CD4 counts after 6 and 36 months of treatment turned this trend on its head. Now, compared with the group starting with 500 CD4s, those starting with 0 to 24 CD4s had half the chance of dying after 36 months (HR 0.52, 95% CI 0.31 to 0.86), and those starting with 50 to 99 cells also had about half the risk of dying after 36 months (HR 0.54, 95% CI 0.33 to 0.87).
Sterne and coworkers stressed that "this does not imply that it is better to initiate cART at lower CD4 cells counts!" (The italics and exclamation point are the authors'.) But what does it mean? Sterne explained that the counterintuitive correlation between lower CD4 count when starting therapy and a higher risk of death after 36 months arises only after statistical adjustment for CD4 count at 36 months. In the researchers' words, "this negative association arises because, for any given CD4 at 36 months, patients with higher CD4 at initiation have benefited less from cART." And it's this reduced benefit that favors a worse prognosis. To put it another way, where a person's CD4s are now is only one bit of datum to consider. How much the CD4s changed over 36 months also matters.
That statistical wrinkle makes sense if one looks at how inflated the death risk becomes when comparing people who reach a count of 500 or more after 36 months of therapy and people with fewer total CD4s at 36 months (Table 2). An analysis adjusted for CD4 counts a month 0 and month 6 showed a significantly higher risk of death for all 36-month CD4 brackets except 350 to 499 cells, and the risk for the 350-to-499-cell group just missed statistical significance (the 95% confidence interval just crosses 1.0).
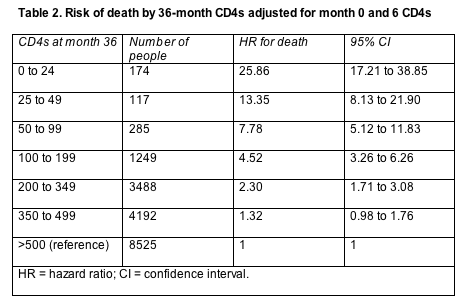
Sterne and collaborators urged clinicians not to forget a person's pretreatment CD4 count when considering progression risk 3 years down the treatment road. That pretreatment count, they explained, "has prognostic value additional to that provided by current CD4 count."
Life expectancy still truncated with HIV in West
Although potent antiretroviral combinations revoked the immediate death sentence of HIV infection, two studies at the Retrovirus Conference showed that life expectancy of infected people in North America and Europe still substantially lags that of people without HIV.
ART Cohort Collaboration researchers figured that a 20-year-old starting therapy in 2003-2005 will not live to celebrate a 54th birthday [4]. Robert Hogg (University of British Columbia) and colleagues based their analysis on more than 26,000 ART-naive people starting their first regimen in three periods--14,993 people starting from1996 through 1999, 9385 from 2000 through 2002, and 3614 from 2003 through 2005. Everyone stayed on treatment for at least 1 year in Europe or North America. Hogg reckoned age-standardized mortality rates for all-cause mortality, life expectancy from exactly 20 years of age, and life expectancy stratified by gender, history of injecting drug use, and CD4 count upon entering the cohort.
Mortality per 1000 people fell over each treatment period, from 45.1 in 1996-1999, to 31.5 in 2000-2002, and to 27.8 in 2003-2005. Those rates proved even lower in 20- to 44-year-olds: 35.7 in 1996-1999, 25.6 in 2000-2003, and 12.7 in 2003-2005. But 20-year-olds in the 1996-1999 cohort could expect to live only another 24.3 years, while those in the 2000-2002 group could look forward to only another 27.1 years of life, and those in the 2003-2005 group could count on only another 33.2 years. Percentages of 20-year-olds who could anticipate living to 44 stood at only 43.8% in 1996-1999 but climbed to 54.1% in 2000-2002 and to 78.6% in 2003-2005.
Reversing trends in the population at large, 20-year-old HIV-infected men could expect to live slightly longer than women--33.5 versus 33.0 years--if they started ART in 2003-2005. Twenty-year-old injecting drug users, on the other hand, could look forward to only another 28.2 years of life if they began treatment in that period versus 34.7 years among non-drug users.
Not surprisingly, life expectancy in the 2003-2005 group rose with each higher CD4-cell bracket at cohort entry: 30.9 years for people with fewer than 200 cells, 33.8 years for those with 200 to 349 cells, and 38.3 years for those with 350 or more cells. But 20-year-olds with an entry CD4 count at or above 350 had blunted life expectancies according to this analysis: Only 76.4% in the 1996-1999 cohort could expect to see their 44th birthday, compared with 75.2% in the 2000-2002 cohort, and 89.6% in the 2003-2005 cohort.
Better therapies of more recent years seem to be helping HIV-infected people catch up with the general population in life expectancy. Still, Hogg and ART Cohort Collaboration coworkers "encourage health planners to use these data to improve health services and living conditions for people living with HIV."
A US study also found curtailed life expectancy in people with HIV, especially minorities and those who get diagnosed with a low CD4 count or discontinue antiretrovirals early [5]. Elena Losina (Massachusetts General Hospital) and colleagues at other centers figured that HIV-infected racial and ethnic minorities lose an average 1.5 years of life compared with whites, while black and Hispanic women lose an average 2.5 years compared with white women.
Losina used a mathematical model incorporating data from the National HIV Research Network to estimate life years lost according to gender and ethnicity and in people who start antiretrovirals with advanced HIV infection or who suspend therapy too early. This cohort is 50% African American and 25% Hispanic. The researchers analyzed three scenarios for starting antiretrovirals--with a CD4 count of 201 to 350 or diagnosis of an opportunist ("early"), with a CD4 count of 51 to 200 ("late"), or with a count of 50 or fewer CD4s ("very late"). Premature discontinuation of antiretroviral therapy meant not proceeding to a second-line regimen after failure of a first regimen.
One third of the National HIV Research Network population fell into the "late" diagnosis group, but 40% to 50% of blacks sought medical care in this "late" stage. One quarter of the overall population never got an HIV diagnosis until they reached the "very late" stage, but blacks and Hispanics were 80% more likely to get a "very late" diagnosis. Up to 14% of the whole population stopped therapy prematurely, but early stopping rates were especially high in black and Hispanic women.
When physicians stick to current US treatment guidelines, Losina's model predicted that HIV-infected people can expect to live 9.6 fewer years than people without HIV. If people start antiretrovirals late or stop early, they stand to lose another 5.1 years of life. A 33-year-old with HIV infection can count on only another 28 years of life, according to Losina's calculations, compared with 44 years for a 32-year-old without HIV.
The model looked at four sequential regimens--first-line efavirenz plus two nucleosides followed by atazanavir/ritonavir plus two nucleosides, lopinavir/ritonavir plus two nucleosides, and darunavir plus enfuvirtide and an optimized background regimen. Thirty-three-year-olds who start antiretrovirals "early" can expect to live another 16, 21, 24, or 26 years depending on how many of those regimens they take. A 33-year-old who starts "late" can expect to live another 14, 18, 20, or 21 years if they take one, two, three, or four of those combinations. And a 33-year-old who starts "very late" can bank on only another 11, 13, 14, or 15 years with that regimen sequence.
The extra years of life lost because of late starting and early stopping proved higher for Hispanic women (6.4 years), black women (5.3 years), and Hispanic men (5.2 years) than for black men (4.8 years), white men (4.2 years), or white women (3.7 years). Prematurely stopping antiretrovirals alone cut an estimated 2.8 years off the lives of Hispanic women and 1.9 years off the lives of black women, compared with 1.1 years for white women.
Coming to medical care late in the course of HIV infection remains a nagging problem in North America and Europe. Jeanne Keruly and Richard Moore from Baltimore's Johns Hopkins Hospital reported that CD4 count at diagnosis has not improved since 1990 in their metropolitan cohort [6]. In fact in all patient groups except gay men, CD4 count at diagnosis fell significantly since 1990. Multivariate analysis showed that older age, male gender, and black race independently raised the risk of coming into care with fewer CD4s.
Keruly and Moore analyzed trends in four groups--1100 people diagnosed in 1990-1994, 927 in 1995-1998, 822 in 1999-2002, and 323 in 2003-2006. No one had antiretroviral experience when they joined the cohort. Median age at diagnosis crept up over the years, from 34 in 1990-1994, to 38 in 1995-1998, 39 in 1999-2002, and 40 in 2003-2006. The proportion of males diagnosed dropped from 66% in the first two periods to 64% in 1999-2002 and to 61% in 2003-2006. The proportion of blacks diagnosed fell from 80% in the first period to 61% in the last period, while the proportion of whites rose from 18% to 39%.
The percentage of people seeking care with an AIDS-defining illness remained fairly stable (and high) over the four periods--37%, 38%, 33%, and 35%. But the first CD4 count dropped sharply for the following groups, though it seemed to rebound in 2003-2006 for some:
CD4 count at HIV diagnosis
- Women: 448 CD4 cells in 1990-1994, 398 in 1995-1998, 312 in 1999-2002, and 302 in 2003-2006
- Men: 335 CD4 cells in 1990-1994, 264 in 1995-1998, 238 in 1999-2002, and 285 in 2003-2006
- Blacks: 361 CD4 cells in 1990-1994, 300 in 1995-1998, 253 in 1999-2002, and 289 in 2003-2006
- Whites: 432 CD4 cells in 1990-1994, 363 in 1995-1998, 318 in 1999-2002, and 317 in 2003-2006
- Heterosexually infected people: 410 CD4 cells in 1990-1994, 327 in 1995-1998, 270 in 1999-2002, and 293 in 2003-2006
- Injecting drug users: 395 CD4 cells in 1990-1994, 331 in 1995-1998, 274 in 1999-2002, and 291 in 2003-2006
Among men who have sex with men, initial CD4 counts appeared to fall and rise again, from 330 in 1990-1994, to 285 in 1995-1998, to 291 in 1999-2002, then back up to 308 in 2003-2006.
For the cohort as a whole, compared with 1990-1994, first CD4 counts were 52 cells lower in 1995-1998 (P = 0.001), 74 cells lower in 1999-2002 (P < 0.001), and 84 cells lower in 2003-2006 (P < 0.001). Every additional 10 years of age correlated with a 17-cell lower count at diagnosis (P = 0.02), male gender meant a 98-cell lower first count (P < 0.001), and black race meant a 74-cell lower first count (P < 0.001).
Keruly and Moore noted that the Baltimore metropolitan area offers free voluntary counseling and HIV testing and that a federal program pays for antiretrovirals regardless of insurance status. But they did not speculate on why CD4 counts at diagnosis have dropped over the years. Growing awareness of long-term side effects in the late 1990s may be one reason. More tolerable regimens in more recent years could contribute to the higher starting CD4 counts in some groups after 2003.
References
1. Grabar S, Lanoy E, Allavena C, et al. Survival after the first AIDS-defining illness and causes of death before and after potent ART: results from the French Hospital Database on HIV. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 525.
2. Lewden C, May T, Rosenthal E, et al. Causes of death among HIV-infected adults in France in 2005 and evolution since 2000. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 976.
3. Sterne J and the ART Cohort Collaboration. Prognosis of patients treated with cART from 36 months after initiation of therapy, according to current and previous CD4 cell count. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 526.
4. Hogg R and the ART Cohort Collaboration. Life expectancy of persons at the time of initiating cART in high-income countries. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 972.
5. Losina E, Schackman B, Sadownik S, et al. Disparities in survival attributable to suboptimal HIV care in the US: influence of gender and race/ethnicity. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 142.
6. Keruly J, Moore R. Immune status at presentation to care has not improved
among ART-naive persons from 1990 until 2006. 14th Conference on Retroviruses and Opportunistic Infections. February 25-28, 2007. Los Angeles. Abstract 975.
|
| |
|
 |
 |
|
|