 |
 |
 |
| |
Predicted Risk of Coronary Heart Disease (CHD) with Tipranavir Exposure Compared to Conventional PI in the RESIST Trial
|
| |
| |
Reported by Jules Levin
CROI, Feb 2007, Los Angeles
N Friis-Moller1, M Kraft2, H Valdez3, D Hall3, and JD Lundgren1
Copenhagen HIV Programme, Hvidovre University Hospital, Hvidovre, Denmark1; Virology, Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany2;Virology, Boehringer Ingelheim Pharmaceuticals, Ridgebury, Ridgefield, Connecticut, USA3
BACKGROUND
The use of Tipranavir (TPV/r) is indicated in treatment-experienced patients
In two pivotal, phase III clinical trials - RESIST-1 and RESIST-2 - treatment-experienced patients with substantial resistance to existing PIs were randomized to receive an optimized background regimen with either ritonavir-boosted tipranavir (TPV/r) or another conventional ritonavir-boosted PI (CPI/r)
TPV/r showed superior treatment responses compared to CPI/r 1
Elevation of lipid levels have been observed more frequently after use of TPV/r (500mg/200mg BID) than after use of other low dose ritonavir boosted PIs
Based on data collected in the RESIST trials, we estimated the potential added risk for coronary heart disease (CHD) associated with TPV/r use compared to CPI/r
METHODS
In treatment experienced patients, mean 5 and 10-year CHD risk was modeled according to metabolic factors observed at baseline and after 8 weeks of starting medication in the RESIST trials
-- Primary emphasis was made to the observations at week 8, as most patients remained adherent to randomized drug assignment at this study visit
The CHD risk estimates were calculated using the Framingham risk equation, a parametric statistical model controlling for multiple cardiovascular risk factors2
-- There are data to suggest that the incremental CHD risk associated with metabolic changes induced by PIs occurs without any substantial delay3, and that
-- The Framingham score fairly reliably predicted the risk of myocardial infarction in HIV-infected individuals4
The following measured covariates were introduced in to the Framingham prediction model:
--Age
--Sex
--Diabetes
--Prior cardiovascular disease
--The ratio of serum Total Cholesterol: HDL Cholesterol (TC:HDL)
--While the following co-variates were unavailable:
--Blood pressure
--Smoking
Therefore, in the primary analyses (A) the prevalence of smoking and the average blood pressure are imputed and takes the average values observed in the D:A:D study3 :
A. Mean systolic blood pressure 120 mmHg, and smoking prevalence 45%
- Different average values were considered in sensitivity analyses in men for the following scenarios:
B. Systolic blood pressure 120 mmHg , smoking prevalence 100%
C. Systolic blood pressure 130 mmHg, smoking prevalence 45%
D. Systolic blood pressure 130 mmHg, smoking prevalence 100%
E. Systolic blood pressure 140 mmHg, smoking prevalence 45%
F. Systolic blood pressure 140 mmHg, smoking prevalence 100%
G. Systolic blood pressure 120 mmHg, smoking prevalence 45%, diabetes prevalence 100%
H. Systolic blood pressure 140 mmHg, smoking prevalence 100%, diabetes prevalence 100%, age 60
The numbers needed to treat to harm one person (NNTH) treated with TPV/r over and above the number of patients experiencing a CHD event on CPI/r were calculated from the absolute CHD risk difference between the study arms:
-- NNTH = 1/absolute risk difference
RESULTS
see tables & graphs below after the text or if not transmitted to you by email this report with all graphs & tables will be posted on the NATAP website in the CROI Conference report section
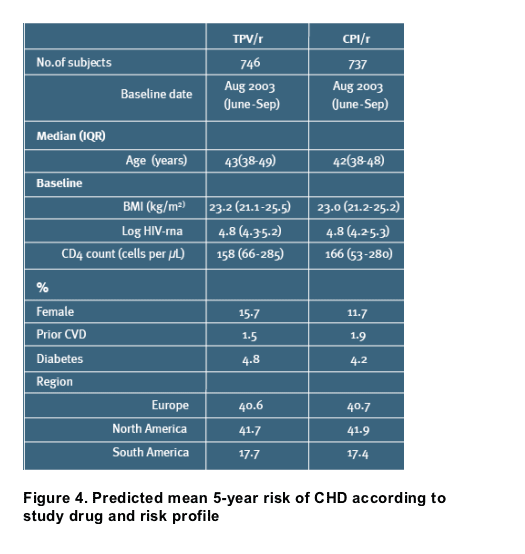
In the RESIST trials, 746 and 737 individuals were randomized to TPV/r and CPI/r, respectively [Table 1]
-- Week 8 data were available for 630 (TPV/r) and 613 (CPI/r) individuals
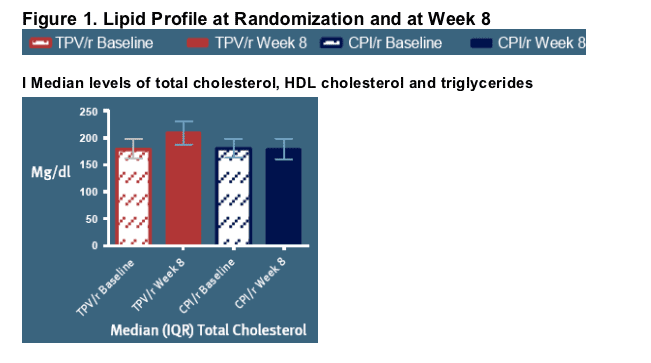
Over the first 8 weeks of exposure, changes in lipid levels were observed [Figure 1, I and II]
-- The TC:HDL ratio increased from 5.1(4.1-6.3) to 6.0 (4.7-7.6) in the TPV/r arm, while from 5.0 (4.1-6.2) to 5.2 (4.1-6.5) in the CPI/r arm (p-value for difference between arms at week 8: <0.0001) [Figure 1, II]
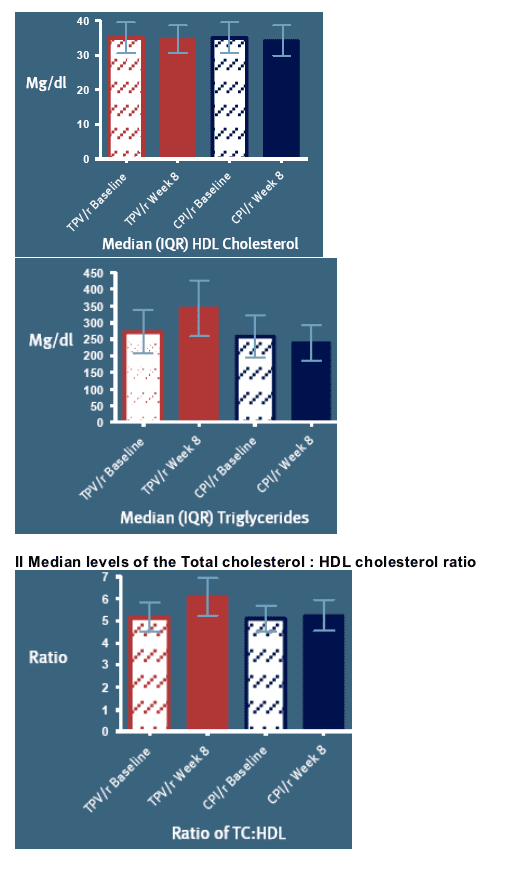
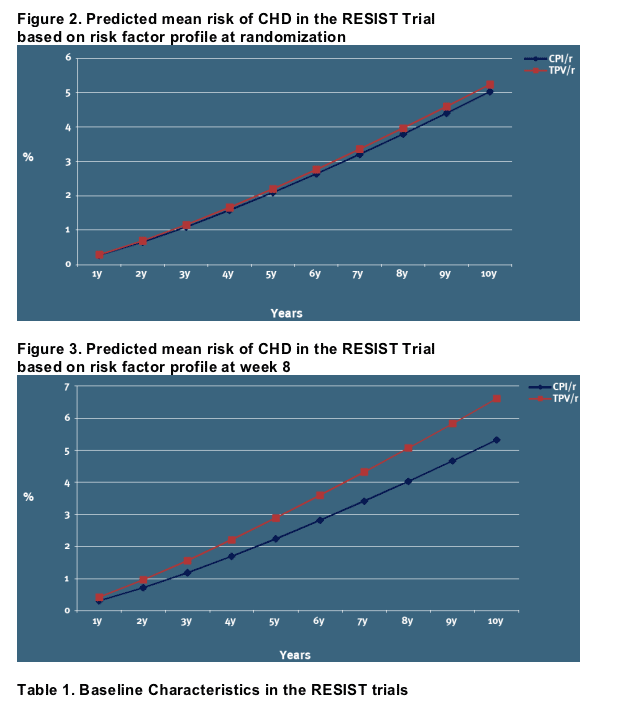
The predicted risk of CHD over a 10-year period is illustrated in Figures 2-3:
Figure 2 shows the predicted risk of CHD based on baseline parameters, and illustrates that - at baseline - this predicted risk is quite similar for both arms
However, at 8 weeks of exposure the modelled CHD risk differ [Figure 3]
The predicted average risk of CHD over a 5-year period in the primary analysis (A) is 2.88% overall in the TPV/r arm versus 2.24% in the CPI/r arm [Figure 3], for a NNTH estimate of 155 (1/(0.0288-0.0224))
That is, to observe one additional CHD event over a 5-year period over and above what would be expected after treatment with CPI/r, 155 patients would need to be treated with TPV/r
However, the absolute risks vary greatly by gender:
-- 3.15% for men, and 1.49% for women in the TPV/r arm versus 2.38% and 1.08% in the CPI/r arm, and thus NNTH is considerably lower for men (131) than women (245)
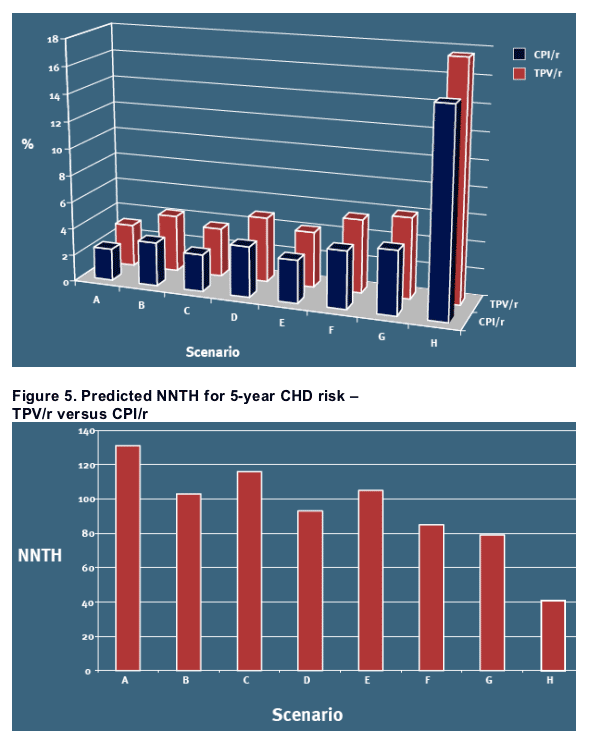
On top of the predictions from the primary analysis, Figures 4 and 5 also includes the sensitivity analyses (B-H) for 5-year predicted CHD risk and NNTH in men
Among the selected scenarios, the highest absolute risk and thus the lowest estimate of NNTH (41) are observed in the analysis to the right [Figures 4 and 5, scenario H], illustrating the risk in diabetic men aged 60 who smokes and have hypertension
CONCLUSIONS
In two large pivotal clinical trial studies conducted in highly treatment experienced patients, TPV/r demonstrated superior treatment response to standard of care PI containing regimens1
A difference in lipid parameters is observed in the RESIST studies by week 8, which translates in to an increased predicted risk of CHD in the TPV/r arm compared to the CPI/r arm
-- However, in absolute terms, the incremental risk of CHD is modest and should be balanced against the beneficial effects of treatment in preventing progression to AIDS:
-- The differences in predicted absolute risks between the TPV/r and CPI/r arms results in estimated NNTH in the range of 80- 150 for the men in the study over a 5-year period, and considerably higher NNTH (i.e. lower absolute risks) for the women in the study
In certain subgroups of patients that present at baseline with well-known cardiovascular disease risk factors, and thus a higher a priori risk of CHD, the estimated NNTH is lower
In aggregate, these findings suggest that in failure patients requiring a boosted PI regimen, the potential added harm on risk of CHD induced by TPV/r versus CPI/r is present but limited in absolute terms in most patients
However, there are groups of patients in whom the absolute risk of CHD over a 5-year period is as high as 15% irrespective of study arm
Thus, prudent evaluation of underlying cardiovascular disease risk factors - and consequently absolute risk of CHD - is important to assure appropriate risk assessment and patient management
COMMENTS
The estimates of CHD risk presented here need to be interpreted with care:
-- Risk estimates were based on the application of a conventional CHD risk equation (the Framingham score), designed for use in the US general population2
-- Although validated in HIV-infected individuals4, a more accurate prediction model may be required for this population
-- Further, the underlying assumption for the present analyses is that the lipid levels observed in week 8 remains stable throughout the ensuing prediction period
References
1.Hicks CB, Cahn P, Cooper DA et al. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for
treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with
Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet 2006; 368(9534):466-475.
2.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991; 121(1 Pt 2):293-298.
3.The D:A:D Study group. Risk of Myocardial Infarction in Association with Different Classes of Antiretroviral Drugs. N Engl J Med 2007; In press.
4.Law MG, Friis-Moller N, El-Sadr WM et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med 2006; 7(4):218-230.
|
| |
|
 |
 |
|
|