| |
Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B
|
| |
| |
Hepatology Feb 2007
Irene Rapti 1, Evangelini Dimou 1 2, Panayota Mitsoula 2, Stephanos J.
Hadziyannis 1 2 *
1Department of Medicine and Liver Unit, Henry Dunant Hospital, Athens, Greece
2Hepatitis Research Laboratory at Evgenidion Hospital, Athens University, Athens, Greece
Abstract
We studied the long-term efficacy of adefovir dipivoxil (ADV) treatment in 42 HBeAg-negative patients with chronic hepatitis B (CHB) who had developed genotypical lamivudine (LAM) resistance with virological and clinical breakthroughs under long-term LAM treatment. Patients were allocated in 2 treatment groups.
In the first (n = 14), LAM was switched to ADV monotherapy whereas in the second (n = 28) ADV was added to LAM. The two groups did not differ in patients' characteristics, all of them having HBV genotype D infection with the precore stop codon mutation.
Within 12 months from start of ADV treatment, serum HBV DNA became nondetectable and ALT normalized in 71% and 90% of patients, respectively, with no difference between the 2 arms. Patients with baseline HBV DNA levels less than 10[7] copies/ml experienced a significantly earlier and more frequent decline in serum HBV DNA to nondetectable levels as compared with patients with greater than 10[7] HBV DNA copies/ml at baseline (P = 0.0013) This response has hitherto been maintained (median treatment duration 40 months) in all patients with ADV added to LAM, whereas virological and biochemical breakthroughs due to development of ADV signature resistance mutations occurred in 3 of 14 patients (21%) on ADV monotherapy 15 to 18 months from start of treatment (P = 0.0174).
Conclusion: Adding ADV to LAM in HBeAg-negative CHB patients with LAM resistance effectively suppresses HBV replication in most of them and induces biochemical remission that can be maintained in all of them at least for 3 years without any evidence of development of resistance to ADV.
Introduction
Chronic infection with the hepatitis B virus (HBV) represents a global health problem, being a major cause of liver disease, morbidity, and mortality. Effective long-term treatment of chronic viral B liver disease improves significantly patients' survival and reduces the risk of development of major complications.[1] Long-term nucleos(t)ide analog administration represents the first-line/first-choice therapy for most patients with chronic hepatitis B (CHB), particularly those with the hepatitis B e antigen (HBeAg)-negative type of the disease.[1-5] Lamivudine (LAM) is the first nucleoside analog approved in the treatment of CHB and, because of potency, safety profile, and relatively low cost, it has been and is still widely applied globally as first-choice therapy for CHB patients either HBeAg-positive or HBeAg-negative.[1] However, approximately 70% of CHB patients in long-term LAM therapy develop resistance to the drug within approximately 3 years of treatment and experience clinical relapses that may be severe and occasionally life threatening.[4][6-9] Continuation of LAM therapy in patients who have developed virologic resistance is of no benefit to them.[7][10]
LAM-resistant patients have been treated with adefovir dipivoxil (ADV) either in monotherapy or in combination with LAM without significant differences between the two regimens at least during the first year of treatment.[11][12] The recently approved nucleoside analog entecavir is also effective; however, entecavir resistance develops in 9% of LAM-resistant patients within 24 months of therapy.[13][14] No data exist on the long-term efficacy of switching from LAM to ADV versus adding ADV in HBeAg-negative LAM-resistant patients.
In our center, an open-label study of long-term LAM treatment in HBeAg-negative CHB patients has been running since 1997. When ADV became first available in an expanded access program, a two-arm study comparing the efficacy and safety of adding ADV in LAM-failing patients versus switching from LAM to ADV was initiated. We report on the long-term efficacy of up to 4 years' duration in the two arms of this ongoing study.
Patients and Methods
Study Population.
Adult patients with HBeAg-negative HBV chronic liver disease participating in an ongoing, previously described protocol of long-term LAM monotherapy[7][8] were included in the current randomized controlled study if they had developed genotypical HBV resistance plus virological and biochemical breakthroughs to LAM. Patients included in the source protocol of long-term LAM treatment were hepatitis B surface antigen (HBsAg)-positive, HBeAg-negative/anti-HBe positive for 6 months or longer, had elevated ALT values in three separate monthly occasions, HBV-DNA greater than 10[5] copies/ml within the last month before starting LAM therapy, and all had compensated liver disease with histological findings of chronic hepatitis either with or without histological evidence of cirrhosis. Patients were excluded if they had antibody to hepatitis C or D virus or to human immunodeficiency virus (HIV), or had received a liver transplant or any antiviral drug other than IFN- in the past. All patients gave written informed consent to participate in these studies. Both study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Long-Term ADV Treatment in Patients Who Developed LAM Resistance.
Forty-two patients who developed virological and clinical resistance to LAM were included in this two-arm treatment protocol of ADV at a 10-mg daily dose. In arm A, LAM treatment was switched to ADV whereas in arm B ADV was added on LAM.
Virological Assays.
Serum HBV-DNA levels were determined by a commercially available quantitative PCR assay (Amplicor HBV Monitor Test, Roche Diagnostics GmbH, Mannheim, Germany) with a sensitivity, according to the manufacturer, of approximately 1,000 copies/ml (cp/ml) as well as by a real time in-house PCR of similar sensitivity. All serum samples with HBV DNA nondetectable by or close to the cutoff level of these PCR assays, were retested by the Cobas TaqMan PCR assay supplied by Roche.[15][16]
Sequencing of the HBV genome for YMDD and ADV mutations was performed at baseline and on any occasion of virological breakthrough under therapy. HBV-DNA extraction, PCR amplification, and sequencing were performed as described in a previous study of our group,[7] using Dye Primer Cycle Sequencing Kit and the Open Gene automated DNA sequencing system (Visible Genetics Inc., Toronto, Canada) according to manufacturer's instructions and primers S1 (5-ATGGAGAACATCACATCAGGA, nt 157-177), POL3 (5-AAGGATCCAGTTGGC, nt 1409-1395), POL14 (5-AGTCCCCAACCTCCA-3, nt 317-332), POL2 (5-ACGGGGTAAAGGTTC-3, nt 1152-1138) and S3 (5-CAAGGTATGTTGCCCGTTTG, nt 455-474). The amplified rt region was approximately 200 codons long covering domains B to D.
HBV precore sequences were determined in serum samples drawn at baseline using primers AC1 (5-CACCTCTGCACGTCGCATGG, nt 1592-1611) and BC1 (5-GGAAAGAAGTCAGAAGGCAA, nt 1974-1955) both for amplification and sequencing. HBV genotypes were also determined at baseline by comparing the obtained S gene nucleotide sequence (nt 477-821) with published sequences.[17]
Follow-up and Definitions.
All patients were followed with monthly clinical examinations and routine laboratory tests. The upper limit of normal for ALT was 49 IU/l. Initial virological response was considered as nondetectable serum HBV-DNA by PCR and initial biochemical response as the decline of ALT within normal range in two consecutive determinations during therapy. Virological breakthrough was considered as the reappearance of detectable serum HBV-DNA by PCR after an initial period of nondetectability, and biochemical breakthrough was defined as the increase of ALT activity above 1.5 X upper limit of normal after an initial return to normal levels.
Statistical Analysis.
All data were analyzed using the statistical package SPSS (version 10.0, SPSS Inc., Chicago, IL). The Mann-Whitney test was used for comparisons of quantitative variables between groups, Wilcoxon matched-pairs signed-ranks test for evaluation of changes of variables within the same group, and the corrected chi-square or 2-tailed Fisher's exact test for qualitative data. The Kaplan-Meier method was used to estimate both virologic and biochemical remission rates during the study period and Cox regression analysis to evaluate the association of several characteristics with the maintenance of remission. In all cases, a 2-tailed P< 0.05 was considered statistically significant.
Results
Biochemical and Virologic Response.
Forty-two LAM-resistant patients were included in the study and were randomly assigned to ADV monotherapy (arm A) or an ADV-LAM combination scheme (arm B) in a 1 to 2 ratio. The baseline characteristics of the studied patients are shown in Table 1. There were no differences in patients' characteristics between the two arms of the study. All were white, had HBV genotype D infection, and all were harboring the precore stop codon mutation with G1896A. Sixteen of the patients had histological evidence of cirrhosis.
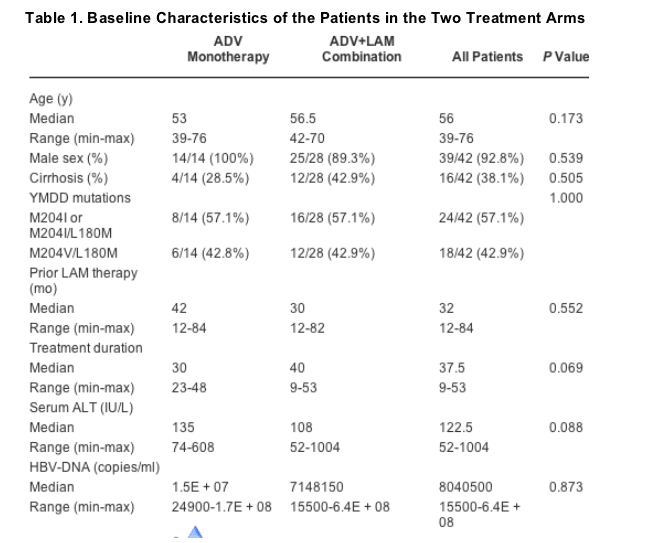
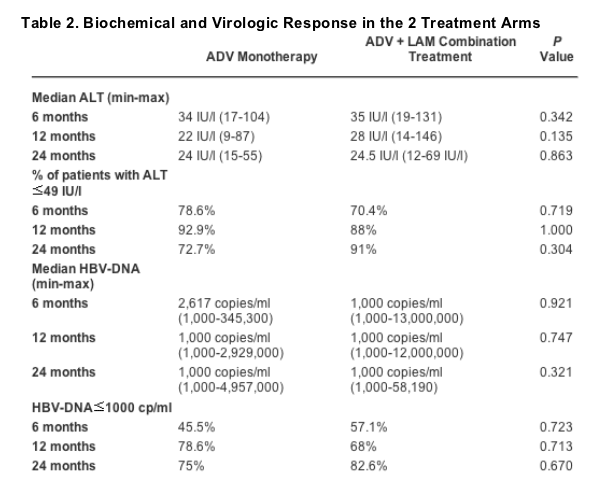
Patients had been on LAM treatment for 12 to 84 months (median, 32 months) before starting ADV and were positive for LAM-resistant signature YMDD mutations (Table 1), whereas none of them had such changes detectable at start of LAM therapy. Serum ALT levels declined under treatment in both arms of the study, with median ALT values declining from 135 IU/l at baseline to 39, 34, 22, and 24 IU/l at months 3, 6, 12, and 24, respectively, in the ADV monotherapy arm and from 108 IU/l at baseline in the combination arm to 39, 35, 28, and 24.5 IU/l at the same times, respectively. Return of serum ALT to normal levels was observed in 57%, 78.6%, 92.9%, and 72.7% of the patients in arm A at 3, 6, 12, and 24 months of treatment, respectively, versus 60.7%, 70.4%, 88%, and 91% of patients in arm B, for the same points in time, respectively. In Fig. 1, the cumulative probability of return of ALT to normal in the 2 arms of treatment is depicted. There were no statistically significant differences between groups. However, biochemical relapse after return of ALT to normal was observed only in the ADV monotherapy arm. No ALT flares were manifested after either switching to or adding ADV to LAM.
Serum HBV DNA levels declined quickly under therapy in both arms without significant differences between ADV monotherapy and combination therapy, at least up to month 12. The cumulative probability of HBV DNA becoming nondetectable under therapy in both arms of the study is shown in Fig. 2. Further analysis of the virological response to treatment according to the baseline HBV DNA levels showed a significantly earlier and more frequent decline to nondetectable levels by PCR among patients with fewer than 107 copies/ml at baseline compared with greater than 10[7] copies/ml. The difference was statistically significant in each arm separately (P = 0.0329 and 0.0185 for arms A and B, respectively) with the P value reaching 0.0013 in the statistical analysis of all patients in the study (Fig. 3). Biochemical and virological responses in both arms of treatment are depicted in Table 2. In both arms, optimal or suboptimal virological responses at month 12 (undetectable HBV DNA or <104 copies/ml, respectively) were more frequent in patients with lower than 107 copies/ml at baseline. Moreover, the hitherto end of treatment virological response rates were found to be statistically different according to this cutoff: HBV-DNA level of 10[4] copies/ml at month 12 (P = 0.04 for arm A, P = 0.009 for arm B and P = 0.0001 for all patients). Neither ALT at baseline (P = 0.697 for arm A, P = 0.487 for arm B, and P = 0.960 for all patients) nor duration of prior LAM treatment (P = 0.83 for arm A, P = 0.77 for arm B, and P = 0.144 for all patients) affected optimal or suboptimal response at month 12.
All patients achieving nondetectability of HBV-DNA also had their ALT levels return to normal, except 1 patient in arm B who, despite complete virologic response with undetectable HBV-DNA from month 15 of treatment, remained with persistently increased ALT activity. This individual underwent a second liver biopsy in the third year of therapy that showed typical changes of steatohepatitis.
HBV Resistance to ADV.
Sequencing of the HBV-DNA under therapy disclosed that LAM-resistant HBV mutations disappeared under ADV monotherapy as well as under combination therapy, except in patients in arm B with suboptimal response to combination therapy not achieving undetectability by PCR of HBV-DNA. Such patients continued to harbor LAM-resistant HBV strains up to the time that HBV-DNA reached nondetectability even after increasing the ADV dose (ADV dose was doubled in two patients). In Table 3,[4][7][18][19] serial genotypic HBV resistance mutation in patients with positive PCR samples during therapy are shown.
All patients with suboptimal response to ADV in arm A (n = 3) had their LAM-resistant HBV mutants reversed to wild-type HBV. All three of them developed subsequently genotypical resistance to ADV at months 15, 18, and 18, respectively, with selection of rtN236T in the first, rtA181V in the second, and a mixed population of rtN236T and rtA181T in the third. Genotypic resistance was followed by both virological and biochemical breakthrough in 2 of them and only by virological breakthrough in the third (mutation A181T+N236T). In all 3 patients, LAM was added as soon as the breakthrough was identified, because adefovir-resistant HBV mutants, particularly rtN236T, have been found to be sensitive to LAM.[18][19]
No evidence of ADV resistance could be detected in arm B with maximal hitherto duration of therapy up to 53 months. Although the number of patients in this study is relatively small, the difference in the cumulative probability of ADV resistance between the 2 treatment arms was found to be statistically significant (P = 0.0182) (Fig. 4).
Side Effects.
Long-term ADV treatment in these LAM-resistant HBeAg(-) patients, either alone or in combination with LAM, was generally well tolerated. No patient in group A has discontinued the drug or reduced the dosage, and none has developed decompensation of cirrhosis or HCC. As far as serious adverse events are concerned, one of the patients underwent gastrectomy due to gastric cancer but remains in good clinical condition with undetectable HBV-DNA.
In the 28 patients in the combination arm, the hitherto median duration of treatment is 40 months (range, 9-53). All patients are still under therapy. In 2 of the 28 patients in the combination arm, both with cirrhosis, the ADV dose had to be reduced to 10 mg every other day, because of a decrease of creatinine clearance from 114 to 50 ml/min and from 71 ml/min to 36 ml/min, respectively.
As far as serious adverse events are concerned, 3 of the 16 patients with cirrhosis (all in group B) developed HCC. Two of them had an optimal and the third had a suboptimal virological response to treatment. In 1 of these patients, HCC was diagnosed in the second year and in the other 2 during the third year of treatment. No statistically significant difference was seen in the rate of HCC development between the 2 arms (P = 0.545). One of these patients underwent chemoembolization and is currently in a transplantation waiting list. The second patient underwent hepatectomy and is in good condition, free of cancer, while continuing combination therapy. The third patient refused any therapeutic modality for HCC but continues antiviral treatment.
Discussion
The results of the current study clearly show that a daily 10-mg dose of ADV in LAM-resistant patients with HBeAg(-) CHB when added to ongoing LAM treatment achieves effective virological suppression associated with biochemical remission in more than 80% without emergence of ADV resistance for up to 3 to 4 years. The efficacy of HBV-DNA suppression in terms of decline to nondetectable levels by sensitive PCR assays at month 12 of therapy was found to be practically the same as in the arm of patients treated with ADV alone. However, after the first year of therapy, 21% of the patients in the monotherapy arm compared with 0% in the combination arm developed genotypic ADV resistance with virological breakthroughs. Despite the relative small number of patients in arm A, the difference is clearly statistically significant (P = 0.0182, Fig. 4). ADV resistance in HBeAg(-) treatment-naive patients with CHB has been observed in less than 2% of such individuals during the second year of ADV monotherapy.[20] A similarly high prevalence of ADV resistance under monotherapy with this drug has recently been reported in LAM-resistant patients with CHB from Asia, most of them HBeAg (+).[21-23] In view of these findings, one may reasonably assume that HBV mutants with changes conferring resistance to LAM develop earlier and more frequent ADV resistance than wild-type HBV strains, similar to what has been found in the case of entecavir therapy for the treatment of LAM-resistant patients.[14] Conversely, the potency of ADV in achieving nondetectability of serum HBV-DNA evaluated at month 12 of therapy does not differ whether the drug is given alone or in combination with LAM and is mainly determined in both groups by HBV-DNA levels at baseline (Fig. 3). Similar observations have recently been reported in Italian CHB patients with LAM resistance[24] as well as in treatment-naive patients under ADV monotherapy with maximal HBV-DNA suppression achieved at month 12 of treatment and being again determined by baseline HBV-DNA levels.[25-27] On the basis of this evidence one may propose that in HBeAg-negative patients, both LAM-resistant and treatment-naive baseline HBV-DNA levels less than 10[7] copies/ml are used as a strong determinant and excellent predictor of response to ADV treatment and achievement of HBV-DNA negativity at month 12. In particular, in clinical practice patients under LAM monotherapy should be strictly monitored for HBV resistance, and ADV should be added on LAM before serum HBV DNA increases to such high levels.
Development of ADV resistance in treatment-naive patients has already been found to be determined by HBV-DNA levels at month 12 of therapy.[27] The same observation has been made in the current study, because all patients in the monotherapy arm who developed ADV resistance had detectable HBV-DNA levels at month 12 (ranging from 9,710 to 2,929,000 copies/ml), whereas all of the other monotherapy patients had undetectable HBV-DNA at the same time. Conversely, although eight patients from the combination arm had also detectable HBV-DNA levels at month 12, none of them developed subsequent ADV resistance. These findings are compatible with the hypothesis that the concomitantly ongoing LAM administration hampers the emergence of ADV-resistant strains and is consistent with in vitro and in vivo data that ADV-resistant HBV mutants are sensitive to LAM.[28][29]
Despite the fact that no ADV-resistant mutations were identified in LAM-resistant patients treated by the combination of ADV with LAM, clearly the efficacy of this therapy was not sufficient to achieve virological and biochemical remission in all patients. In all such patients, however, continuing detectability of HBV DNA was always found to be due to persistence of the LAM-resistant strains of HBV (Table 3). The most probable explanation for this appears to be the relative low potency of the 10-mg daily dose of ADV, which was actually selected not for its robustness but because of safety reasons.[26] Therefore, to deal with inadequate potency of ADV, either its dose should be increased or another drug of similar resistance profile but of higher potency against LAM-resistant mutants should be substituted. Tenofovir fumarate, to which LAM-resistant HBV strains are also sensitive and which is much more potent than ADV,[31][32] is an excellent candidate for such cases. The optimal time for a change to this compound could be month 12 of treatment. In the current study, in those patients with persistent HBV DNA detectability beyond year 3, the ADV dose was increased to 20 mg/day with good response, without side effects, followed by significant decline of HBV DNA levels to close to nondetectability.
In conclusion, the high rate of HBV-DNA nondetectability by the combination of ADV and LAM therapy in month 12 in LAM-resistant HBeAg(-) patients in this study, combined with the absence of ADV resistance for up to 4 years of ongoing therapy, makes reasonable the recommendation of adding ADV while continuing LAM, as the first-line, first-choice therapy for this group of patients.
|
|
| |
| |
|
|
|