| |
Predicting response to peginterferon a-2a (Pegasys), lamivudine and the two combined for HBeAg-negative chronic hepatitis B; genotype may predict response to combination therapy
|
| |
| |
Gut May 2007;56:699-705
F Bonino1, P Marcellin2, G K K Lau3, S Hadziyannis4, R Jin5, T Piratvisuth6, G Germanidis7, C Yurdaydin8, M Diago9, S Gurel10, M-Y Lai11, M R Brunetto12, P Farci13, M Popescu14 and P McCloud15 for the Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group
1 University of Pisa and Foundation IRCCS, Policlinico di Milano, Milan, Italy
2 Service d'Hepatologie, INSERM Unite 481 and Centre de Recherches Claude Bernard sur les Hepatite Virales, Hopital Beaujon, Clichy, France
3 Queen Mary Hospital, The University of Hong Kong, Hong Kong, China
4 Department of Medicine and Hepatology, Henry Dunant Hospital, Athens, Greece
5 Digestive Disease Department, Beijing You An Hospital, Beijing, China
6 Department of Medicine, Prince of Songkla University, Songkla, Thailand
7 Pathology Clinic, Papageorgiou General Hospital, Thessalonika, Greece
8 Faculty of Medicine, University of Ankara, Ankara, Turkey
9 Hospital General Universitario de Valencia, Valencia, Spain
10 University of Uludag, Faculty of Medicine, Bursa, Turkey
11 Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan, ROC
12 Azienda Ospedaliera Universitaria Pisana, Pisa, Italy
13 Universita di Cagliari, Cagliari, Italy
14 Roche, Basel, Switzerland
15 Roche, Dee Why, Australia
ABSTRACT
Objective: In a trial of patients with hepatitis B e antigen (HBeAg)-negative chronic hepatitis B, 24 week post-treatment biochemical and virological response rates with peginterferon -2a with or without lamivudine were significantly higher than with lamivudine alone. The effect of pre-treatment factors on post-treatment responses was investigated.
Methods: Multivariate analyses were performed using available data from 518 patients treated with peginterferon -2a with or without lamivudine, or with lamivudine alone. A post-treatment response was defined as alanine aminotransferase (ALT) normalisation and hepatitis B virus (HBV) DNA level of <20 000 copies/ml.
Results:
In logistic regression analyses across all treatment arms, peginterferon a-2a (with or without lamivudine) therapy, younger age, female gender, high baseline ALT, low baseline HBV DNA and HBV genotype were identified as significant predictors of combined response at 24 weeks post-treatment.
In the peginterferon a-2a and lamivudine monotherapy arms, patients with genotypes B or C had a higher chance of response than genotype D infected patients (p<0.001), the latter responding better to the combination than to peginterferon a-2a monotherapy (p = 0.015).
At 1 year post-treatment, response rates by intention-to-treat analysis were 19.2% for the peginterferon a-2a, 19.0% for the combination, and 10.0% for the lamivudine groups, with genotypes B or C associated with a sustained combined response to peginterferon a-2a with or without lamivudine therapy.
Conclusions:
Baseline ALT and HBV DNA levels, patient age, gender, and infecting HBV genotype significantly influenced combined response at 24 weeks post-treatment, in patients treated with peginterferon a-2a and/or lamivudine. At 1 year post-treatment HBV genotype was significantly predictive of efficacy for patients treated with peginterferon a-2a with or without lamivudine.
Chronic hepatitis B virus (HBV) infection continues to be a major global health problem with more than 400 million cases worldwide and is a significant cause of cirrhosis and hepatocellular carcinoma.1 Hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) represents the late phase of the infection where the HBV population is predominantly made up of HBV precore/basic core promoter variants that reduce or abolish the expression of HBeAg.2,3 Spontaneous sustained remissions are rare in patients with HBeAg-negative CHB and the disease is characterised by persistent viral replication, progressive liver damage and early development of cirrhosis.4-6 HBeAg-negative CHB is particularly prevalent in the Mediterranean region and Asia. Nevertheless, recent studies indicate that HBeAg-negative disease may be more common than previously suspected and that its prevalence is increasing worldwide.2,3
Until recently, conventional interferon-a and lamivudine were the only drugs specifically licensed for the treatment of CHB. However, in patients with HBeAg-negative CHB, both drugs are associated with high rates of relapse upon treatment discontinuation and poor sustained response rates.7,8,9,10,11 Furthermore, conventional interferon has a suboptimal pharmacokinetic profile that necessitates an inconvenient dosing regimen (three times weekly). Lamivudine is hindered by the development of drug resistance, which increases with prolonged use.12,13 In the past few years, the nucleotide analogue, adefovir, has been approved as a treatment option for CHB, although continuous administration is required in patients with HBeAg-negative disease due to rapid biochemical, virological, and histological rebound upon adefovir withdrawal.14 Continuous adefovir therapy is also associated with increased drug resistance, albeit at a lower cumulative rate than lamivudine.15 Peginterferon a-2a, which was also recently approved for the treatment of CHB, has been shown to provide significantly higher post-treatment response rates compared with lamivudine in patients with HBeAg-negative CHB.16 Peginterferon a-2a, which has dual immunomodulatory and antiviral activity, has also proved more effective than both conventional interferon and lamivudine in patients with HBeAg-positive disease.17,18
To date, investigations of predictors of response have generally focused on patients with HBeAg-positive CHB. In patients with this phase of the disease, low serum HBV DNA levels and high serum alanine aminotransferase (ALT) levels at baseline have been shown to be independently associated with improved responses to conventional interferon .19-22 Likewise, in patients receiving lamivudine, high baseline ALT was identified as the most important predictor of HBeAg loss by multivariate modelling.23 Higher rates of HBeAg clearance after conventional interferon therapy have been reported for Asian patients infected with HBV genotype B compared with genotype C.22,24 However, HBV genotype seems not to influence HBeAg responses to lamivudine, at least in Chinese patients.25 At present, no detailed analysis of predictors of response has been performed in patients with HBeAg-negative CHB. Accordingly, in the current study we report predictors of combined response to peginterferon a-2a monotherapy, peginterferon a-2a plus lamivudine combination therapy, and lamivudine monotherapy using data from a large multinational, randomised trial involving 537 patients with HBeAg-negative disease.16
DISCUSSION
The current study describes the first detailed analysis of predictors of response in HBeAg-negative CHB. In multivariate analyses of patients treated with peginterferon a-2a alone, lamivudine alone, or the two in combination, high baseline ALT, low baseline HBV DNA, younger age and female gender were identified as significant predictors of a combined ALT and HBV DNA response at 24 weeks post-treatment. The multivariate models also showed that response rates, both at 24 weeks and at 1 year post-treatment, were significantly higher with peginterferon a-2a monotherapy than with lamivudine monotherapy, even after adjusting for the effect of the relevant baseline factors.
Baseline ALT levels had a strong effect on combined response after 48 weeks of treatment and at 24 week post-treatment follow-up, with patients in the highest baseline ALT category (>5xULN) achieving the highest response rates, regardless of which treatment they received. This effect was particularly noticeable with the peginterferon a-2a-containing regimens, with almost one half of the patients with baseline ALT >5xULN achieving a combined response at 24 weeks post-treatment. The effect of lower baseline HBV DNA levels on combined response was most evident in the peginterferon -2a plus lamivudine and lamivudine monotherapy arms. Brunetto et al have previously identified three biochemical profiles in HBeAg-negative CHB: (i) intermittent ALT flares with intervening periods of normal liver enzyme; (ii) continuous ALT elevation without flares; and (iii) intermittent ALT flares superimposed on continuous ALT elevation.6
The higher rate of response seen in patients with elevated ALT at the time of treatment initiation in our study suggests that biochemical reactivation of disease activity may be a suitable time for starting therapy in such patients, and should be explored further. The data indicate that in patients with HBeAg-negative CHB and elevated ALT (with the possible exception of those with compensated cirrhosis), treatment with peginterferon a-2a is both efficacious and safe, provided that the patients are carefully selected and monitored throughout treatment.
In this study, combined response rates at 24 weeks post-treatment were consistently higher in female patients compared with male patients. This was most apparent in those treated with combination therapy or lamivudine alone. When combined response rates were stratified by patient age, the highest rates were seen in younger patients, irrespective of which treatment they received. This finding supports data from a previous study suggesting that younger patients treated with conventional interferon have a higher probability of survival compared with older patients.28
Genotype was also identified as a significant predictor of combined response. The influence of genotype was particularly apparent in patients treated with peginterferon a-2a or lamivudine monotherapy, with those infected with HBV genotype B or C having higher rates of combined response compared with those infected with genotype D. In contrast to the overall findings of the study, which did not document a benefit of the combination regimen over peginterferon a-2a monotherapy, the combination of peginterferon a-2a and lamivudine seems to be beneficial to patients with genotype D. Genotype D patients receiving combination therapy had substantially higher rates of combined response than genotype D patients receiving peginterferon -2a monotherapy. This apparent benefit of the combination regimen versus peginterferon -2a monotherapy was not seen in patients infected with the other genotypes. However, this observation agrees with those of a recently published study by Colombatto et al29 where bio-mathematical modelling revealed a greater reduction in HBV-infected cells at the end of therapy among patients with genotype D who were receiving combination therapy.
Since only a small number of patients infected with genotype D participated in the long-term section of the study, it was not possible to confirm the benefits of the combination regimen in this subgroup of patients at the 1 year post-treatment time point. In summary, as these findings are based on relatively few patients and could be the result of random variation, additional comparative data are required to confirm if combination therapy is beneficial in the subset of HBeAg-negative CHB patients specifically infected with HBV genotype D.
Analysis of the subset of patients who achieved a virological response (an HBV DNA level of <20 000 copies/ml) at the end of treatment with peginterferon a-2a (with or without lamivudine) revealed that older patients and those with higher baseline and/or end-of-treatment HBV DNA levels were more likely to experience relapse than younger patients or those with lower HBV DNA levels. Our finding that higher baseline and end-of-treatment HBV DNA levels were associated with relapse concurs with previous studies of conventional interferon alone or in combination with lamivudine.9,30 In these patients with virological response at the end of treatment, HBV genotype was also seen to influence the durability of response to peginterferon a-2a-containing therapy with patients infected with HBV genotype D more likely to relapse post-treatment than genotype C infected patients.
One of the limitations of our study is that predictor analysis using the entire study population was only possible based on efficacy outcomes assessed at 24 weeks post-treatment. An analysis of the efficacy results at 1 year post-treatment was based on a subgroup of patients participating in the long-term study, and those not participating were regarded as non-responders. For this reason, it cannot be ruled out that a selection bias might have affected the results seen in this subpopulation at this time point post-treatment.
In conclusion, the current analyses show that baseline ALT and HBV DNA levels, as well as patient age and gender, are factors which might influence post-treatment response. HBV genotype emerges as a new predictive factor in HBeAg-negative CHB, with genotype D being associated with a poorer response to treatment than other genotypes. With verification in future trials, these data on predictors of sustained response in patients with HBeAg-negative CHB may prove useful in clinical practice by allowing the selection of the most appropriate treatment strategy based on individual patient characteristics. The role of HBV genotype in tailoring treatment regimens deserves further investigation.
RESULTS
Effect of pre-treatment factors on combined response at 24 weeks post-treatment (data from the initial study)
As previously reported,16 the percentage of patients achieving a combined response at 24 weeks post-treatment was significantly higher with peginterferon a-2a monotherapy (36%; 63/177) or peginterferon a-2a plus lamivudine combination therapy (38%; 68/179) than with lamivudine monotherapy (23%; 42/181; p = 0.011 and p = 0.002, respectively).
Of the 537 patients analysed using the intent-to-treat principle in the original study,16 only those of Asian or Caucasian origin and singularly infected with HBV genotype A, B, C or D were included in the overall logistic regression analysis (n = 518); 19 patients were excluded as they were of non-Caucasian/non-Asian origin and/or were infected with a mixed HBV genotype population or HBV genotypes E, G or H.
Multivariate analyses
In the initial multivariate analysis of individual pre-treatment factors, baseline HBV DNA, baseline ALT, treatment arm, age and gender were significant predictors of a combined response in patients receiving peginterferon a-2a monotherapy, peginterferon -2a plus lamivudine, or lamivudine monotherapy; genotype, ethnicity, body weight and screening ALT were not significant. Because ethnicity and genotype were highly correlated and could potentially have a confounding effect on one another, multivariate analysis was performed with ethnicity excluded from the logistic regression model. The baseline variables found to be significant predictors of response in the initial model (ie, baseline HBV DNA, baseline ALT, age, gender and type of treatment) remained significant (table 1).
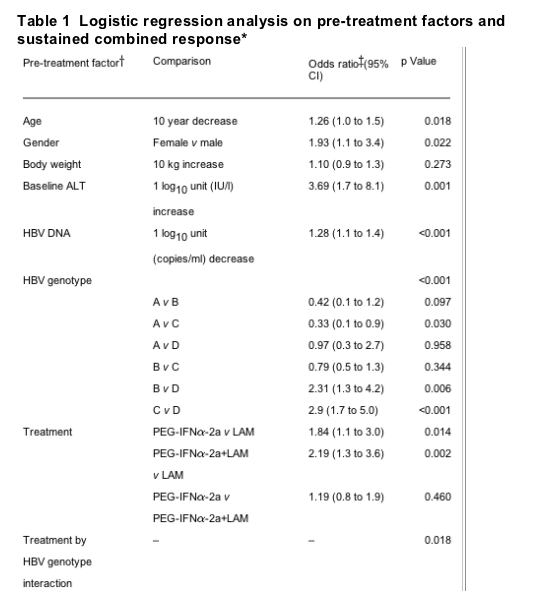
*Combined ALT normalisation and an HBV DNA level of <20 000 copies/ml after 48 weeks of treatment and 24 weeks of treatment-free follow-up; ALT at screening was included in the model but was not a significant predictor of response (p = 0.952); each odds ratio adjusted for other variables in the model.
In addition, genotype was also identified as a significant predictor of combined response at 24 weeks post-treatment (p<0.001). The odds ratios from the logistic regression analysis indicated that a 10-fold higher baseline ALT level (ie, 500 v 50 IU/l) increased the odds of combined response 3.7-fold, and that female patients had around twice the odds of achieving a combined response compared with male patients. The multivariate analysis also confirmed that the rate of combined response was significantly higher with peginterferon a-2a monotherapy (p = 0.014; odds ratio (OR): 1.84) and peginterferon a-2a plus lamivudine (p = 0.002; OR: 2.19) than with lamivudine, even after adjusting for the effect of the other pre-treatment variables included in the model. Thus, those patients treated with peginterferon a-2a (with or without lamivudine) had around twice the odds of achieving a combined response at 24 weeks post-treatment compared with those treated with lamivudine monotherapy.
Of the 518 patients evaluated in the overall logistic regression model, 400 patients had available baseline HAI scores. Using data from these 400 patients in a separate multivariate analysis including all prospectively defined pre-treatment variables except ethnicity, baseline HAI score was not a significant predictor of combined response at 24 weeks post-treatment (p = 0.936).
Stratified analysis by significantly predictive factors
As shown in fig 1A, rates of combined response tended to be higher in patients with higher baseline ALT levels, regardless of treatment. The influence of baseline ALT on combined response was particularly noticeable in the two peginterferon a-2a-containing arms, with a combined response occurring in almost half of the patients with ALT values >5xULN. While HBV DNA was a significant predictor in the multivariate analysis, baseline HBV DNA was not a significant predictor by univariate analysis (p = 0.088) (fig 1B). Female and male patients responded equally well to peginterferon a-2a monotherapy (fig 1C). However, in the combination therapy and lamivudine monotherapy arms, combined response rates were higher in female patients than in male patients. Patients under 40 years of age had higher combined response rates than patients over 40 years of age, regardless of which treatment they received (fig 1D). Patients treated with peginterferon a-2a (with or without lamivudine) achieved higher rates of combined response than patients treated with lamivudine monotherapy, irrespective of baseline ALT, HBV DNA, gender or age.
Effect of HBV genotype on combined response at 24 weeks post-treatment
In the overall logistic regression model, genotype was identified as a significant predictor of combined response by multivariate analysis (p<0.001) (table 1). Furthermore, in this model, a statistically significant interaction between treatment arm and HBV genotype was identified (p = 0.018). This interaction indicated that the response to treatment was not uniform across the four HBV genotypes A, B, C and D, and that patterns of response to the three study drugs differed according to genotype.
When the logistic regression analysis was conducted in a subgroup of 346 patients receiving either peginterferon a-2a monotherapy or lamivudine monotherapy, the interaction between treatment arm and genotype was no longer significant (p = 0.637). This model therefore indicated that rates of combined response were higher with peginterferon a-2a than with lamivudine, regardless of genotype (p = 0.012; OR: 1.9; 95% CI: 1.2 to 3.2). The multivariate analysis also showed that genotype clearly influenced response in patients treated with peginterferon a-2a or lamivudine monotherapy, as the chance of a combined response was greater in patients infected with genotypes B or C versus genotype D (p<0.001; OR: 5.9; 95% CI: 2.7 to 13.1, and p<0.001; OR: 4.6; 95% CI: 2.2 to 9.5, respectively). This finding was also reflected in stratified analyses where patients infected with genotype D had substantially lower rates of combined response than patients with genotypes B or C (16% (9/55) v 44% (19/43) and 49% (31/63) with peginterferon a-2a; 11% (7/63) v 39% (19/49) and 26% (15/57) with lamivudine, respectively).
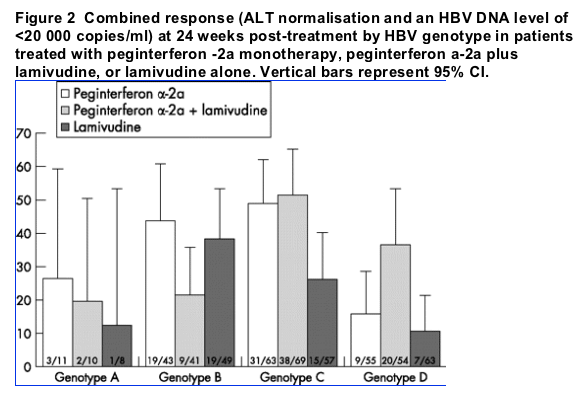
When the multivariate model was conducted in 342 patients treated with peginterferon a-2a monotherapy or peginterferon plus lamivudine, the interaction between treatment arm and genotype remained significant (p = 0.027). This interaction occurred because of a different pattern of response to the two peginterferon a-2a regimens among patients infected with genotypes B or D (fig 2); in patients with genotype B the rates of combined response were 44% for peginterferon a-2a monotherapy and 22% for peginterferon a-2a plus lamivudine, while in patients with genotype D the rates of combined response were 16% for peginterferon a-2a monotherapy and 37% for peginterferon a-2a plus lamivudine. After adjusting for the effects of gender, age, body weight, screening ALT, baseline ALT and baseline HBV DNA, the comparison of peginterferon a-2a plus lamivudine versus peginterferon a-2a monotherapy in HBV genotype D infected patients resulted in an OR of 3.5 (95% CI: 1.3 to 9.1; p = 0.015). In patients with HBV genotype B, the same comparison resulted in an OR of 0.4 (95% CI: 0.1 to 1.2; p = 0.097).
Factors associated with sustained virological response and post-treatment virological relapse in patients receiving peginterferon -2a-containing treatment
Comparative analyses were carried out using data from 294 of 308 patients treated with peginterferon a-2a (with or without lamivudine) who achieved a virological response (an HBV DNA level of <20 000 copies/ml) at the end of the 48 week treatment period; 14 patients of non-Asian or non-Caucasian origin and/or not singularly infected with HBV genotype A, B, C or D were excluded from the analyses. In total, 139 (47%) of these 294 patients had a sustained HBV DNA level of <20 000 copies/ml at 24 weeks post-treatment; the remaining 155 patients had an HBV DNA level of >20 000 copies/ml at 24 weeks post-treatment and were considered to have had a virological relapse. Table 2 shows pre-treatment and on-treatment characteristics for patients with a sustained virological response versus those with virological relapse. Logistic regression showed that patient age, HBV genotype and HBV DNA level at the end of treatment (week 48) were significantly associated with post-treatment durability of response by univariate analysis. In the multivariate analysis, age, baseline and end-of-treatment viral load, and HBV genotype were the only factors significantly influencing post-treatment durability of response. This means that a sustained virological response to peginterferon a-2a (with or without lamivudine) was more likely in younger patients (p<0.024; OR: 1.3 per 10 year decrease; 95% CI: 1.0 to 1.7), patients with lower baseline HBV DNA levels (p<0.01; OR: 1.2 per 1 log decrease; 95% CI: 1.0 to 1.4), and patients with lower end-of-treatment HBV DNA levels (p<0.029; OR: 2.9 per 1 log decrease; 95% CI: 1.1 to 7.7). In addition, the multivariate analysis showed that genotype C infected patients had a higher chance of a sustained virological response after peginterferon a-2a (with or without lamivudine) therapy than genotype D infected patients (p<0.001; OR: 3.3; 95% CI: 1.7 to 6.5).
Effect of pre-treatment factors on combined response at 1 year post-treatment (data from the long-term follow-up study)
In total, 304 of the original 537 patients (57%) analysed in the initial study participated in the roll-over observational study to assess the durability of response to treatment, including 111 (63%) of those originally treated with peginterferon a-2a monotherapy, 111 (62%) of those treated with peginterferon a-2a plus lamivudine, and 82 (45%) of those treated with lamivudine alone.27
As with the patients analysed at 24 weeks post-treatment, only those infected with HBV genotypes A, B, C and D were included in the analysis at the later time of assessment. The rates of combined response observed at 1 year post-treatment were similar to those measured at 24 weeks post-treatment in all three treatment groups who participated in the long-term study. The proportion of patients achieving an HBV DNA level of <20 000 copies/ml and normal ALT at 1 year post-treatment was 33.3% (34/102; 95% CI: 24.3 to 43.4) for those originally in the peginterferon monotherapy group, 32.7% (34/104; 95% CI: 23.8 to 42.6) for those in the peginterferon+lamivudine combination therapy group, and 24.0% (18/75; 95% CI: 14.9 to 35.3) for those in the lamivudine group. Applying intention-to-treat (ITT) analysis, where patients with missing data at the 1 year follow-up were deemed to be non-responders, the response rates for the three treatment groups were 19.2% (34/177; 95% CI: 13.1 to 25.3), 19.0% (34/179; 95% CI: 13.0 to 25.0) and 10% (18/181; 95% CI: 5.3 to 14.6), respectively.
Data from the multivariate analysis using the original ITT population confirmed that a combined virological and biochemical response at 1 year post-treatment was more likely to be achieved with peginterferon a-2a monotherapy (p = 0.0223; OR 2.11) and peginterferon a-2a with lamivudine (p = 0.0185; OR 2.16) than with lamivudine monotherapy (table 3). These odds ratios indicated that patients treated with peginterferon a-2a with or without lamivudine had approximately twice the potential of achieving a combined response at 1 year post-treatment than did those treated with lamivudine alone. In addition, genotype was a significant predictor of response at 1 year post-treatment in logistic regression analysis. Also, patients infected with HBV genotypes B (p = 0.0033) or C (p<0.0001) had a better chance of achieving a combined response at 1 year post-treatment than did genotype D infected patients (table 3). On the other hand, age, gender, weight, baseline ALT and baseline HBV DNA were not significant predictors of combined response to treatment at 1 year after treatment cessation (table 3).
METHODS
The patients and results of the study of peginterferon a-2a monotherapy versus peginterferon a-2a plus lamivudine versus lamivudine monotherapy in HBeAg-negative CHB have been described elsewhere.16 All patients gave written informed consent. All patients were positive for hepatitis B s antigen (HBsAg) and anti-HBe antibody, but negative for HBeAg. Entry criteria included an HBV DNA level of >100 000 copies/ml and an ALT level >1 times but <10 times the upper limit of normal (ULN; 30 IU/l in this study). All included patients had CHB status confirmed by liver biopsy within the 12 months prior to randomisation. Previous treatment for CHB was allowed, but not within the 6 months prior to the study. Patients were randomly assigned in a 1:1:1 ratio to receive 48 weeks of either peginterferon a-2a (40KD; PEGASYS, Roche, Basel, Switzerland) 180 ƒÊg once weekly plus oral placebo once daily; peginterferon a-2a 180 ƒÊg once weekly plus lamivudine 100 mg once daily (Epivir-HBV/Zeffix, GlaxoSmithKline, Greenford, UK); or lamivudine 100 mg once daily. The co-primary endpoints of the study were ALT normalisation and an HBV DNA level of <20 000 copies/ml, measured at 24 weeks post-treatment (week 72). All study centres involved in the initial clinical trial were invited to participate in a roll-over long-term observational study to assess the durability of response. For this analysis, patients were assessed 1 year after the end of treatment using the same efficacy measures as at 24 weeks post-treatment.
Virological, biochemical and histological measurements
Serum HBV DNA was measured at a central laboratory using the COBAS AMPLICOR HBV MONITOR (Roche Diagnostics, Branchburg, NJ, USA). Serum ALT was measured at local laboratories in accordance with common standardised procedures. Baseline biopsy specimens were scored using the modified Histological Activity Index (HAI) score26 by an independent histopathologist who was unaware of the patient's treatment assignment.
Definition of response and relapse
In the present analyses, a post-treatment combined response was defined as both ALT normalisation and an HBV DNA level of <20 000 copies/ml, 24 weeks after the end of the treatment period. Patients with missing values at 24 weeks post-treatment were considered as treatment failures (ie, no combined response). A sustained virological response was defined as an HBV DNA level of <20 000 copies/ml at the end of treatment and at 24 weeks post-treatment; post-treatment virological relapse was defined as HBV DNA level of <20 000 copies/ml at the end of treatment but >20 000 copies/ml at 24 weeks post-treatment.
Statistics
Multiple logistic regression with prospectively defined covariates of interest was used to determine pre-treatment factors predictive of a combined response at 24 weeks post-treatment. We did not formally use any of the following automatic selection procedures: forward-entry, backward elimination, stepwise or best-subset selection. The predictors included in the model were all pre-specified, except that "region" was eliminated because of the confounding with genotype. The parameters of the logistic regression were fitted with the maximum likelihood procedure, and all statistical tests were Wald tests.
The pre-treatment factors included were age, gender, genotype, ethnicity, body weight, HAI score, serum ALT (screening and baseline) and serum HBV DNA (baseline). Gender, genotype and ethnicity were included as categorical variables; age, body weight, HAI score, serum ALT and serum HBV DNA were included as continuous variables. Those pre-treatment factors found to be significant in the overall logistic regression model were assessed in stratified analyses to show rates of response across different categories of these characteristics. Additional comparative logistic regression analyses were performed to investigate pre-treatment and on-treatment factors associated with sustained virological response and post-treatment virological relapse. The statistical analysis software used was SAS version 8.0 (SAS Institute, Cary, NC).
|
|
| |
| |
|
|
|