| |
Detection of Hepatitis C Virus (HCV) in Serum and Peripheral-Blood Mononuclear Cells from HCV-Monoinfected and HIV/HCV- Coinfected Persons
|
| |
| |
The Journal of Infectious Diseases July 15, 2005;192:258-265
Jason T. Blackard,1 Laura Smeaton,2 Yoichi Hiasa,6 Norio Horiike,6 Morikazu Onji,6 Denise J. Jamieson,3 Irma Rodriguez,4 Kenneth H. Mayer,4,5 and Raymond T. Chung1
1Gastrointestinal Unit, Massachusetts General Hospital, and 2Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, Massachusetts; 3Division of Reproductive Health, Centers for Disease Control and Prevention, Atlanta, Georgia; 4Department of Medicine, Miriam Hospital, and 5Department of Medicine, Brown University, Providence, Rhode Island; 6Third Department of Internal Medicine, Ehime University School of Medicine, Ehime, Japan
note from Jules Levin: there is quite a bit of controversy regarding whether extra-hepatic (outside the liver) HCV reservoirs exist, and if they are meaningful. The contrvoserial studies found HCV in reservoirs of patients years after achieving SVR. Many researchers think the methods used to detect viral replication outside the liver in those studies are questionable. Most of the limited research has been conducted in HCV monoinfected patients. Here is a study conducted in coinfected patients that has a hint of reliability, and a second similar study to follow. This is not, however, a study of patients who achieved SVR, just a study of coinfected women that finds negative-strand HCV (that suggests viral replication) outside the liver and serum and in PBMCs, a potential extra-hepatic reservoir. The author says to be cautious in interpreting the findings and says the findings 'suggest' HCV replication outside the liver.
"......Our findings regarding detection of positive- and negative-strand HCV RNA in the PBMC compartment by the strand-specific rtPCR assay were strongly suggestive of extrahepatic HCV replication.....Results from our pilot study should be interpreted with caution, given its limited sample size. Nonetheless, we here report several novel findings regarding extrahepatic HCV replication. First, rates of detection of HCV RNA in the PBMC compartment were higher for HIV/HCV-coinfected women than for HCV-monoinfected women..... Importantly, negative-strand HCV RNA, indicative of active viral replication, was detected at higher rates in the PBMC compartments of HIV/HCV-coinfected women....low-level HCV replication in the PBMC compartment, as indicated by detection of negative-strand HCV RNA, may adversely influence the effectiveness of HCV antiviral therapies [38, 42], particularly in HIV/HCV-coinfected persons..."
ABSTRACT
It has been speculated that hepatitis C virus (HCV) replicates in peripheral-blood mononuclear cells (PBMCs), which, therefore, may be a site for interaction with human immunodeficiency virus (HIV). We used strand-specific real-time polymerase chain reaction to detect HCV RNA in 28 HCV-monoinfected and 20 HIV/HCV-coinfected women.
At the first visit, positive-strand HCV RNA was detected in serum samples from 89% of the women, whereas positive-strand HCV RNA was detected in PBMC samples from 32% and 55% of the HCV-monoinfected and HIV/HCVcoinfected women, respectively.
After initiation of antiretroviral therapy, the HIV/HCV-coinfected women were significantly more likely to have detectable positive- and negative-strand HCV RNA in the PBMC compartment than were the HCV-monoinfected women.
HIV and HCV RNA levels were not correlated. Serum HCV RNA levels were correlated over time; HCV RNA levels in the serum and PBMC compartments were not. These data suggest differential regulation of HCV RNA in the serum and PBMC compartments and may partially explain the limited HCV antiviral response rates observed in coinfected persons.
Background
Hepatitis C virus (HCV) is a positive-strand RNA virus that infects >170 million people worldwide. Because of the inability to infect small animals with HCV and the lack of efficient cell-culture models, much of the current understanding of the HCV life cycle has been inferred from studies that use samples from infected humans. Although hepatocytes are the major site of infection, there is a broad clinical spectrum of disease and extrahepatic complications, including cryoglobulinemia, non-Hodgkin lymphoma, and porphyria cutanea tarda [1]. Some studies have reported evidence for extrahepatic replication of HCV in peripheral-blood mononuclear cells (PBMCs); however, these studies have typically involved a small number of patients and have often yielded contradictory results [27]. Other studies have reported evidence for HCV replication in granulocytes, monocytes/macrophages, dendritic cells, and B lymphocytes, as well as in extrahepatic tissues [8-16]. Because certain amplification methods lack strand specificity, which may influence the reliable detection of replication intermediates (i.e., negative-strand HCV RNA), it has been challenging to definitively demonstrate extrahepatic HCV replication. Recently, modification of the real-time polymerase chain reaction (rtPCR) assay to include the Tth enzyme, which has high strand specificity and independent reverse-transcriptase and DNA-dependent polymerase activity, has been used to detect negative-strand HCV RNA in the liver and/or PBMC compartment [7, 9, 17-19].
In the United States, 150,000-300,000 people are coinfected with HCV and HIV [20]. Multiple studies have demonstrated the adverse effects of HIV coinfection on liver fibrosis, HCV RNA levels, HCV disease progression [21], and treatment response rates [22-24]. The mechanisms by which these 2 viruses interact remain unclear, because no direct virus-virus interactions have been demonstrated to date. However, recent in vitro data suggest that HCV and HIV proteins cooperatively induce hepatic apoptotic pathways [25, 26] and secretion of proinflammatory cytokines [27] without requiring cell infection and viral replication. Thus, it is reasonable to speculate that similar signaling cascades in PBMCs may also permit indirect interactions between HCV and HIV.
We have previously investigated serum HCV diversity in HIV/HCV-coinfected persons initiating antiretroviral therapy (ART) for HIV infection [28]. We found significant evolution of the hypervariable region 1, but not the adjacent envelope 1 region, after ART initiation. However, few studies have addressed the effects of ART on HCV in HIV/HCV-coinfected persons in compartments other than serum. The demonstration of extrahepatic HCV replication in the PBMC compartment would have important implications for transmission of the virus and efficient treatment of HCV infection. Nonetheless, previous studies have not assessed this phenomenon in HIV/HCVcoinfected persons in a longitudinal manner, nor have they addressed it in coinfected persons initiating ART [15, 29, 30]. Therefore, we sought to investigate whether HCV replication could be detected in the serum and PBMC compartments of persons coinfected with HIV and HCV and to assess the effect of ART on extrahepatic HCV replication.
PARTICIPANTS, MATERIALS, AND METHODS
Study population. From April 1993 to February 1995, the HIV Epidemiology Research (HER) Study, a prospective natural-history study of HIV infection, enrolled 871 HIV-infected women and 439 demographically matched HIV-uninfected women [31]. The women participated in clinic visits at 6-month intervals through 1999. By study design, one-half of the women reported injection drug use (IDU), and the other half reported only sexual risk behavior.
As described elsewhere, HCV serostatus was determined by either Abbott HCV EIA (version 2.0) or Ortho HCV ELISA (version 3.0) [32]. Overall, the seroprevalence of HCV was 56.5%, with rates of 48.0% and 60.8% in HIV-uninfected and HIV-infected women, respectively. Of the women who acknowledged prior IDU, 88.3% were HCV seropositive; of these women, 76.9% had detectable HCV RNA [33]. Because the HER Study cohort was formed before the widespread use of combination therapy, only 30% were receiving ART at the beginning of the study. By 1999, 31.3% were still not receiving any ART [34].
HER Study participants were included in the present study if they (1) were HCV seropositive, regardless of their HIV status; (2) had serum and PBMC samples available from at least 2 consecutive study visits conducted at the Providence, RI, site; and (3) were not receiving ART at the beginning of the study (for the HIV-infected women). For the HIV-infected women, study visits corresponded to the visit immediately before ART initiation (denoted "visit A") and the visit immediately after ART initiation (denoted "visit B"). The median intervals between visits were 5.8 months and 6.6 months for the HCV-monoinfected and HIV/HCVcoinfected women, respectively. The drug regimens initiated by the HIV-infected women were as follows: >2 nucleoside reverse-transcriptase inhibitors (NRTIs) (n = 4); >1 NRTI plus >1 protease inhibitor (PI) (n = 11); >2 NRTIs plus 1 nonnucleoside reverse-transcriptase inhibitor (NNRTI) (n = 4); and 2 NRTIs plus 1 NNRTI plus 2 PIs (n = 1). One HIV/HCV-coinfected woman was missing serum samples at both visits, and 3 HIV/HCV-coinfected women were missing PBMC samples at visit B.
Cellular RNA extraction and strand-specific Tth rtPCR. RNA was extracted from serum samples by use of the QIAamp Viral RNA Kit (Qiagen). For PBMC samples, the number of cells available was limited. Because the number of cells varied per sample (range, 1.4-7.6 X 106 cells/mL), we normalized all quantitative HCV RNA data on the PBMC compartment to the copy number of a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Five hundred microliters of a PBMC suspension was washed with diethopyrocarbonate (DEPC)treated dH2O, and cellular RNA was extracted by use of TRIzol (Invitrogen). The resultant RNA was resuspended in 40 L of DEPC-treated dH2O and treated 2 times with DNase I (Ambion). Positive- and negative-strand HCV cDNAs were quantified by a validated strand-specific rtPCR assay using SYBR green dye I, as described elsewhere [17, 19]. Extracted RNA was heated at 95C for 1 min and then incubated at 70C. A mixture containing 10 pmol/L HCV-1 antisense primer (5-TGGATGCACGGTCTACGAGACCTC-3; nt 342320, according to the numbering of H77 [35]; GenBank accession number AF009606) for HCV positive-strand synthesis or HCV-2 sense primer (5-CACTCCCCTGTGAGGAACT-3; nt 3856) for HCV negative-strand synthesis, 1X reverse-transcriptase buffer, 1 mmol/L MnCl2, 200 mmol/L each deoxynucleoside triphosphate, and 5 U of Tth enzyme (Applied Biosystems) was added. The cDNA reaction consisted of an annealing step for 2 min at 60C, followed by an extension step for 20 min at 70C. To inactivate the reverse-transcriptase activity of the Tth enzyme, chelating buffer was added after cDNA synthesis. cDNA was purified by use of the High Pure PCR Template Preparation Kit (Roche Diagnostics).
Positive- and negative-strand HCV PCR amplification was performed with 2uL of purified cDNA in a mixture containing LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics), 4 mmol/L MgCl2, and 5 pmol/L each antisense primer KY78 (5-CTCGCAAGCACCCTATCAGGCAGT-3; nt 311288) and sense primer KY80 (5-GCAGAAAGCGTCTAGCCATGGCGT-3; nt 68-91). The PCR consisted of an initial denaturation step for 10 min at 95C, then 40 cycles under the following conditions: 15 s at 95C, 5 s at 70C, and 15 s at 72C. For generation of GAPDH mRNA, cDNA synthesis was performed with an oligo d(T) primer under standard conditions. For PCR amplification, we used a commercial GAPDH primer set (Roche Search LC), with the conditions recommended by the manufacturer.
For each PBMC sample, we determined the positive- and negative-strand HCV RNA copy numbers and normalized them to the GAPDH copy number, to provide standardized values (i.e., positive-strand HCV RNA copies and negative-strand HCV RNA copies per molecule of GAPDH). Serum HCV quantities were expressed as HCV RNA copies per microliter (extracted from 140 L of serum). Previous studies have reported very low rates of negative-strand HCV RNA detection in serum [11, 14, 15]; thus, we did not systematically measure negative-strand HCV RNA in this compartment. To avoid potential cross-contamination, samples for each time point and each compartment from an individual were handled separately. Additionally, all rtPCR amplifications included a negative control that contained no template.
Statistical analyses. Demographic and clinical data were compared by Fisher's exact test for categorical variables and either Student's t test or the Wilcoxon rank sum test for continuous variables. The Wilcoxon rank sum test was used to compare HCV RNA levels between the HCV-monoinfected and HIV/HCVcoinfected women; values for undetectable levels were set at 0 for serum samples (log10 transformed) and at 0.01 for PBMC samples (untransformed). Spearman's correlation test was used to investigate the linear relationships between CD4 cell count, plasma HIV RNA level, and serum and PBMC HCV RNA levels. All P values reported are 2-sided; P < .05 was considered to be statistically significant. No adjustments were made for multiple comparisons. All analyses were performed by use of SAS software (version 9; SAS Institute).
RESULTS
Study cohort characteristics. Twenty-eight HCV-monoinfected and 20 HIV/HCV-coinfected women from the HER Study cohort were selected for the present study. These 2 groups of women did not differ with respect to the reporting of IDU as the main risk factor for HCV acquisition, age at enrollment, or HCV genotype; however, HIV/HCVcoinfected women were more likely to be black (table 1). None of the women reported receiving HCV treatment during the visits included in the present study. Mean CD4 cell counts were lower in the HIV/HCVcoinfected women than in the HCV-monoinfected women at both time points (visit A, 285 vs. 1139 cells/uL [P < .0001]; visit B, 376 vs. 1101 cells/uL [P < .0001]). After ART initiation (between visits A and B), median plasma HIV RNA levels decreased, from 4.1 to 2.1 log10 copies/mL, in the HIV/HCV-coinfected women (P = .0002), whereas mean CD4 cell counts increased, from 285 to 376 cells/uL (P = .4), in these women.
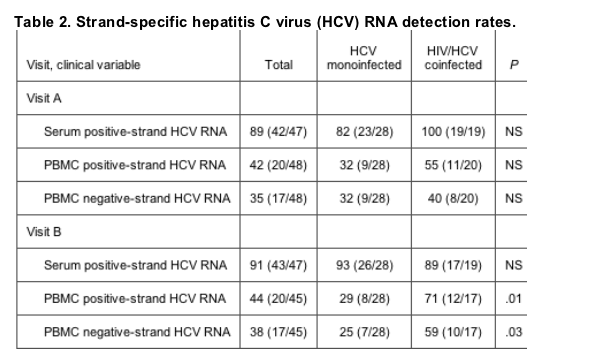
Strand-specific HCV RNA detection rates. At visit A, positive-strand HCV RNA was detected, by a strand-specific rtPCR assay, in serum from 42 (89%) of 47 women, including 23 (82%) of 28 HCV-monoinfected women and 19 (100%) of 19 HIV/HCV-coinfected women (table 2). At visit B, 43 (91%) of 47 women had detectable levels of positive-strand HCV RNA in serum. Rates of detection in the serum compartment were not significantly different between the HCV-monoinfected and the HIV/HCV-coinfected women at either visit. We did not systematically measure levels of negative-strand HCV RNA in the serum compartment. However, of 47 women tested, 34 (72%) had undetectable or negligible levels (<1000 copiesu/L) of negative-strand HCV RNA (data not shown). Furthermore, among those women in whom both strands were detected, the ratio of negative-strand : positive-strand HCV RNA in the serum compartment was <1% in both groups, suggesting that there is a vast excess of positive-strand HCV RNA in the serum compartment (data not shown). In contrast, the proportion of negative-strand HCV RNA relative to positive-strand HCV RNA in the PBMC compartment was significantly higher (particularly in the HIV/HCV-coinfected women), a finding that is consistent with higher rates of HCV replication in the PBMC compartment.
Our findings regarding detection of positive- and negative-strand HCV RNA in the PBMC compartment by the strand-specific rtPCR assay were strongly suggestive of extrahepatic HCV replication. At visit A, positive-strand HCV RNA was detected in the PBMC compartments of 20 (42%) of 48 women, including 9 (32%) of 28 HCV-monoinfected women and 11 (55%) of 20 HIV/HCV-coinfected women. Negative-strand HCV RNA was detected in 17 (35%) of 48 women, including 9 (32%) of 28 HCV-monoinfected women and 8 (40%) of 20 HIV/HCV-coinfected women. At visit B, after the HIV-infected women had initiated ART, positive-strand HCV RNA was still more readily detected in the HIV/HCV-coinfected women than in the HCV-monoinfected women (8/28 [29%] vs. 12/17 [71%]; P = .01). The negative-strand HCV RNA detection rate was also significantly different in the HCV-monoinfected and the HIV/HCV-coinfected women at visit B (7/28 [25%] vs. 10/17 [59%]; P = .03). Negative-strand HCV RNA was detected in the PBMC compartment only when positive-strand HCV RNA was also detected.
Strand-specific HCV RNA levels. Strand-specific HCV RNA levels were determined in the serum and PBMC compartments and, in the latter case, were normalized to the GAPDH copy number (table 3). Using dilutions of serum samples for which HCV RNA levels had previously been determined (by use of the Roche Amplicor Monitor Kit), we determined that the lower level of detection for the strand-specific rtPCR assay was about 260 copies/uL. The HIV/HCV-coinfected women had higher positive-strand HCV RNA levels in serum than did the HCV-monoinfected women, both before and after ART initiation (visit A, 3.6 vs. 5.2 log10 copies/L [P = .002]; visit B, 4.4 vs. 5.5 log10 copies/uL [P = .003]). Because of the low rates of detection of HCV RNA in the PBMC compartment, medians could not be defined in several instances; therefore, 75th percentiles are presented in table 3. At visit A, there was no significant difference in either positive- or negative-strand HCV RNA levels in the PBMC compartment between the 2 groups. At visit B, after ART initiation, both positive- and negative-strand HCV RNA levels in the PBMC compartment were higher in the HIV/HCV-coinfected women than in the HCV-monoinfected women, but these differences did not reach statistical significance.
Correlation analyses. We also analyzed potential correlations between specific immunologic parameters and strand-specific HCV RNA levels (table 4). Age and plasma HIV RNA levels were not correlated with either positive- or negative-strand HCV RNA levels in either compartment. CD4 cell count was inversely correlated with positive-strand HCV RNA levels in the serum (P <.0001), but not in the PBMC, compartment. Positive-strand HCV RNA levels in the serum compartment were consistent over time (P < .0001), as were both positive- and negative-strand HCV RNA levels in the PBMC compartment (P < .0001). However, in the absence of ART, positive-strand HCV RNA levels in the serum compartment were not correlated with either positive- or negative-strand HCV RNA levels in the PBMC compartment. Between visits, neither positive- nor negative-strand HCV RNA levels in the PBMC compartment were correlated.
DISCUSSION
Researchers have long sought to establish whether HCV replicates outside the liver, because detection of HCV RNA in extrahepatic reservoirs has important implications for transmission, disease progression, and effective treatment. Nonetheless, achieving a definitive demonstration of extrahepatic HCV replication has been limited by several biological and technical considerations. Foremost, the lack of a robust cell-culture system has made it exceedingly difficult to compare HCV replication in different cell populations. To date, the dynamics of HCV replication have typically been examined by intensive study of serum-specific or liver-specific HCV RNA; however, viral replication in such extrahepatic reservoirs as PBMCs may not reflect replication in these other compartments. Furthermore, although detection of positive-strand HCV RNA cannot distinguish between nucleic acids participating in replication and those already incorporated into viral particles, detection of replication intermediates, such as negative-strand HCV RNA, is a more biologically relevant measure of active virus replication. Negative-strand HCV RNA is generally present at levels 10-100-fold lower than those of positive-strand HCV RNA [36, 37]; thus, highly sensitive and specific detection assays must be used. Although distinguishing between positive- and negative-strand HCV RNA is critical, not all strand-specific detection methods have high specificity for detection of negative-strand HCV RNA. Here, we have used a validated strand-specific rtPCR assay that includes the Tth enzyme. Because this enzyme contains separate reverse transcriptase and DNA-dependent polymerase functions, it is highly specific and is ideal for discriminating between positive- and negative-strand RNA [17, 19].
Although several studies have measured HCV replication in the PBMC compartment, only a subset have used a bona fide Tth-based amplification assay to distinguish between positive- and negative-strand HCV RNA [4, 9, 17, 29, 3841]. Our rate of detection of negative-strand HCV RNA in the PBMC compartment was somewhat elevated, compared with the results of these previous studies. Such differences could reflect minor discrepancies in the amplification assay, study populations, HCV antiviral receipt, and/or sample preparation. However, it has previously been demonstrated that both HIV coinfection and testing of multiple extrahepatic samples are associated with an increased likelihood of detection of negative-strand HCV RNA [29, 38, 41]. For example, Laskus et al. demonstrated the presence of negative-strand HCV RNA in 5 of 14 PBMC samples from HIV/HCVcoinfected patients [29]. The authors also suggested that factors governing HCV replication at hepatic and extrahepatic sites may differ. Thus, one might anticipate increased detection of negative-strand HCV RNA in a population such as ours, because no participant received HCV antiviral therapy, a high prevalence of HIV coinfection existed, and we tested multiple samples for each participant. It is also theoretically possible that our use of an all-female cohort is responsible for increased detection of negative-strand HCV RNA, although sex-specific detection rates have not been reported to date.
Our study design has several distinct advantages over those of previously published studies. First, to date, most studies of extrahepatic replication have been restricted to a small population analyzed in a cross-sectional, rather than a longitudinal, manner. Second, paired serum and PBMC samples have not usually been analyzed, making intercompartment comparisons difficult. Third, despite clinical data suggesting that HIV adversely affects HCV replication, disease progression, and treatment response rates, HCV-monoinfected and HIV/HCV-coinfected persons have not typically been analyzed as distinct groups. Fourth, not all previously published studies used a strand-specific rtPCR assay that had high strand specificity.
The present study design does have several limitations. First, very low levels of negative-strand HCV RNA were detected in the serum compartments of a subset of women. Because "naked" negative-strand HCV RNAs are not known to circulate outside of cells, we suggest that these very low levels of negative-strand RNA likely represent a small amount of contaminating RNA from residual PBMCs that were not completely removed during the initial processing of whole blood. Second, given the limited number of PBMCs available, we were not able to more precisely define the cell population(s) within PBMCs that are responsible for HCV replication. Nonetheless, there is growing evidence that HCV may infect several peripheral-blood cell types, including B lymphocytes, granulocytes, monocytes/macrophages, and dendritic cells [8, 12, 40]; it is, however, important to note that each of these previous studies either excluded persons coinfected with HIV or did not report HIV status.
Results from our pilot study should be interpreted with caution, given its limited sample size. Nonetheless, we here report several novel findings regarding extrahepatic HCV replication. First, rates of detection of HCV RNA in the PBMC compartment were higher for HIV/HCV-coinfected women than for HCV-monoinfected women. Previous studies have suggested that serum HCV RNA levels are higher in HIV/HCV-coinfected persons [21]; however, this phenomenon has not been investigated in the PBMC compartment until now. Importantly, negative-strand HCV RNA, indicative of active viral replication, was detected at higher rates in the PBMC compartments of HIV/HCV-coinfected women, highlighting an important interaction between these 2 viruses in this compartment. Second, there was no correlation between plasma HIV RNA levels and positive- or negative-strand HCV RNA levels in either the serum or PBMC compartment. Moreover, ART initiation appeared to have a minimal effect on HCV detection rates and HCV RNA levels, although, because of the limited number of HIV/HCV-coinfected persons included in the present study, we cannot rule out a possible association. The finding of elevated HCV RNA levels in the serum and PBMC compartments even after ART initiation may suggest that immune reconstitution after suppression of HIV is not sufficient to control HCV replication. Third, there was an inverse correlation between CD4 cell counts and positive-strand HCV RNA levels in the serum, but not the PBMC, compartment (i.e., as the CD4 cell count increased, the serum, but not the PBMC, HCV RNA level decreased). Given that several components of PBMCs may support HCV replication [8, 12, 40], it is provocative to speculate that the PBMC compartment may be a site in which HCV is partially protected from adaptive and/or innate immune responses. Fourth, there was a positive correlation between positive- and negative-strand HCV RNA levels in the PBMC compartment. However, serum and PBMC HCV RNA levels did not correlate with each other. Thus, HCV RNA may be regulated differently in these compartments.
The precise mechanisms by which HIV influences extrahepatic HCV replication have yet to be determined. It is possible that HIV-induced immunosuppression results in less immunologic control of HCV replication, although reproducible correlations between HCV RNA levels in the serum compartment and CD4 cell counts have not been confirmed [30]. Moreover, the presence of replicative viral forms in extrahepatic sites does not correlate with CD4 cell count [29]. Interestingly, in the present study, HCV RNA levels in the PBMC compartment did not correlate with CD4 cell counts, although positive-strand HCV RNA levels in the serum compartment and CD4 cell counts were inversely correlated. These data imply that immunosuppression alone is not the sole driving force behind increased detection of HCV RNA in the PBMC compartment. It is also possible that HIV, through the induction of interferon antagonists, blunts host innate antiviral responses that would otherwise inhibit HCV replication. HIV may also render specific types of PBMCs more susceptible to HCV infection and replication [41].
In summary, low-level HCV replication in the PBMC compartment, as indicated by detection of negative-strand HCV RNA, may adversely influence the effectiveness of HCV antiviral therapies [38, 42], particularly in HIV/HCV-coinfected persons. Furthermore, the PBMC compartment may be a privileged site for HCV that is capable of reinitiating viral replication after termination of HCV treatment, when conditions once again become more favorable. Thus, even if clearance of HCV from hepatocytes is achieved by treatment, reinfection from such extrahepatic sites as the PBMC compartment may occur [43]. Future studies of HCV quasispecies diversification in serum and PBMCs may provide additional evidence that HCV replicationand evolutionis distinct in these compartments and may require targeted therapeutic approaches.
References
1. Gumber S, Chopra S. Hepatitis C: a multifaceted diseasereview of extrahepatic manifestations. Ann Intern Med 1995; 123:61520. First citation in article | PubMed
2. Maggi F, Fornai C, Vatteroni ML, et al. Differences in hepatitis C virus quasispecies composition between liver, peripheral blood mononuclear cells and plasma. J Gen Virol 1997; 78:15215. First citation in article | PubMed
3. Shimizu YK, Igarashi H, Kanematu T, et al. Sequence analysis of the hepatitis C virus genome recovered from serum, liver, and peripheral blood mononuclear cells of infected chimpanzees. J Virol 1997; 71:576973. First citation in article | PubMed
4. Laskus T, Radkowski M, Wang LF, Cianciara J, Vargas H, Rakela J. Hepatitis C virus negative strand RNA is not detected in peripheral blood mononuclear cells and viral sequences are identical to those in serum: a case against extrahepatic replication. J Gen Virol 1997; 78:274750. First citation in article | PubMed
5. Navas S, Martin J, Quiroga JA, Castillo I, Carreno V. Genetic diversity and tissue compartmentalization of the hepatitis C virus genome in blood mononuclear cells, liver, and serum from chronic hepatitis C patients. J Virol 1998; 72:16406. First citation in article | PubMed
6. Maggi F, Fornai C, Morrica A, et al. Divergent evolution of hepatitis C virus in liver and peripheral blood mononuclear cells of infected patients. J Med Virol 1999; 57:5763. First citation in article | PubMed
7. Okuda M, Hino K, Korenaga M, Yamaguchi Y, Katoh Y, Okita K. Differences in hypervariable region 1 quasispecies of hepatitis C virus in human serum, peripheral blood mononuclear cells, and liver. Hepatology 1999; 29:21722. First citation in article | PubMed
8. Lerat H, Rumin S, Habersetzer F, et al. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood 1998; 91:38419. First citation in article | PubMed
9. Laskus T, Radkowski M, Wang LF, Nowicki M, Rakela J. Uneven distribution of hepatitis C virus quasispecies in tissues from subjects with end-stage liver disease: confounding effect of viral adsorption and mounting evidence for the presence of low-level extrahepatic replication. J Virol 2000; 74:10147. First citation in article | PubMed
10. Caussin-Schwemling C, Schmitt C, Stoll-Keller F. Study of the infection of human blood derived monocyte/macrophages with hepatitis C virus in vitro. J Med Virol 2001; 65:1422. First citation in article | PubMed
11. Radkowski M, Wilkinson J, Nowicki M, et al. Search for hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: evidence of replication. J Virol 2002; 76:6008. First citation in article | PubMed
12. Goutagny N, Fatmi A, De Ledinghen V, et al. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis 2003; 187:19518. First citation in article | Full Text | PubMed
13. Radkowski M, Bednarska A, Horban A, et al. Infection of primary human macrophages with hepatitis C virus in vitro: induction of tumour necrosis factor- and interleukin 8. J Gen Virol 2004; 85:4759. First citation in article | PubMed
14. Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology 1998; 28:13981401. First citation in article | PubMed
15. Laskus T, Radkowski M, Wang LF, Jang SJ, Vargas H, Rakela J. Hepatitis C virus quasispecies in patients infected with HIV-1: correlation with extrahepatic replication. Virology 1998; 248:16471. First citation in article | PubMed
16. Roque-Afonso AM, Jiang J, Penin F, et al. Nonrandom distribution of hepatitis C virus quasispecies in plasma and peripheral blood mononuclear cell subsets. J Virol 1999; 73:921321. First citation in article | PubMed
17. Lanford RE, Chavez D, Chisari FV, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol 1995; 69:807983. First citation in article | PubMed
18. Jang SJ, Wang LF, Radkowski M, Rakela J, Laskus T. Differences between hepatitis C virus 5 untranslated region quasispecies in serum and liver. J Gen Virol 1999; 80:7116. First citation in article | PubMed
19. Castet V, Fournier C, Soulier A, et al. Alpha interferon inhibits hepatitis C virus replication in primary human hepatocytes infected in vitro. J Virol 2002; 76:818999. First citation in article | PubMed
20. Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS clinical trials groups. Clin Infect Dis 2002; 34:8317. First citation in article | Full Text | PubMed
21. Sulkowski M, Thomas D. Hepatitis C in the HIV-infected person. Ann Intern Med 2003; 138:197207. First citation in article | PubMed
22. Chung RT, Andersen J, Volberding P, et al. Peginterferon alpha-2a plus ribavirin versus interferon alpha-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med 2004; 351:4519. First citation in article | PubMed
23. Perez-Olmeda M, Nunez M, Romero M, et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. AIDS 2003; 17:10238. First citation in article | PubMed
24. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004; 351:43850. First citation in article | PubMed
25. Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis 2003; 188:1192204. First citation in article | Full Text | PubMed
26. Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virusinduced apoptosis of human hepatocytes via CXCR4. J Infect Dis 2003; 188:145560. First citation in article | Full Text | PubMed
27. Balasubramanian A, Ganju R, Groopman J. HCV and HIV envelope proteins collaboratively mediate IL-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem 2003; 278:3575566. First citation in article | PubMed
28. Blackard JT, Yang Y, Bordoni P, et al. Hepatitis C virus (HCV) diversity in HIV-HCVcoinfected subjects initiating highly active antiretroviral therapy. J Infect Dis 2004; 189:147281. First citation in article | Full Text | PubMed
29. Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. The presence of active hepatitis C virus replication in lymphoid tissue in patients coinfected with human immunodeficiency virus type 1. J Infect Dis 1998; 178:118992. First citation in article | PubMed
30. Bare P, Massud I, Belmonte L, et al. HCV recovery from peripheral blood mononuclear cell culture supernatants derived from HCV-HIV co-infected haemophilic patients with undetectable HCV viraemia. Haemophilia 2003; 9:598604. First citation in article | PubMed
31. Smith DK, Warren DL, Vlahov D, et al. Design and baseline participant characteristics of the Human Immunodeficiency Virus Epidemiology Research (HER) Study: a prospective cohort of human immunodeficiency virus infection in US women. Am J Epidemiol 1997; 146:45969. First citation in article | PubMed
32. Stover CT, Smith DK, Schmid DS, et al. Prevalence of and risk factors for viral infections among human immunodeficiency virus (HIV)infected and high-risk HIV-uninfected women. J Infect Dis 2003; 187:138896. First citation in article | Full Text | PubMed
33. Thomas DL, Rich JD, Schuman P, et al. Multicenter evaluation of hepatitis C RNA levels among female injection drug users. J Infect Dis 2001; 183:9736. First citation in article | Full Text | PubMed
34. Mayer KH, Hogan JW, Smith D, et al. Clinical and immunologic progression in HIV-infected US women before and after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2003; 33:61424. First citation in article | PubMed
35. Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 1997; 277:5704. First citation in article | PubMed
36. Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Lack of evidence of hepatitis G virus replication in the livers of patients coinfected with hepatitis C and G viruses. J Virol 1997; 71:78046. First citation in article | PubMed
37. Lanford RE, Sureau C, Jacob JR, White R, Fuerst TR. Demonstration of in vitro infection of chimpanzee hepatocytes with hepatitis C virus using strand-specific RT/PCR. Virology 1994; 202:60614. First citation in article | PubMed
38. Radkowski M, Gallegos-Orozco JF, Jablonska J, et al. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology 2005; 41:10614. First citation in article | PubMed
39. Goutagny N, Vieux C, Decullier E, et al. Quantification and functional analysis of plasmacytoid dendritic cells in patients with chronic hepatitis C virus infection. J Infect Dis 2004; 189:164655. First citation in article | Full Text | PubMed
40. Boisvert J, He XS, Cheung R, Keeffe EB, Wright T, Greenberg HB. Quantitative analysis of hepatitis C virus in peripheral blood and liver: replication detected only in liver. J Infect Dis 2001; 184:82735. First citation in article | Full Text | PubMed
41. Laskus T, Radkowski M, Jablonska J, et al. Human immunodeficiency virus facilitates infection/replication of hepatitis C virus in native human macrophages. Blood 2004; 103:38549. First citation in article | PubMed
42. Gong GZ, Lai LY, Jiang YF, He Y, Su XS. HCV replication in PBMC and its influence on interferon therapy. World J Gastroenterol 2003; 9:2914. First citation in article | PubMed
43. Saleh MG, Tibbs CJ, Koskinas J, et al. Hepatic and extrahepatic hepatitis C virus replication in relation to response to interferon therapy. Hepatology 1994; 20:13991404. First citation in article | PubMed
|
|
| |
| |
|
|
|