| |
Impact of Ribavirin Dose Reductions in Hepatitis C Virus Genotype 1 Patients Completing Peginterferon Alfa-2a/Ribavirin Treatment
|
| |
| |
Clinical Gastroenterology & Hepatology
Volume 5, Issue 1, Pages 124-129 (January 2007)
K. Rajender Reddy_1, Mitchell L. Shiffman7, Timothy R. Morgan5, Stefan Zeuzem, Stephanos Hadziyannis6, Fayez M. Hamzeh#3, Teresa L. Wright 4, Michael Fried2
_ University of Pennsylvania, Philadelphia, Pennsylvania
Virginia Commonwealth University, Richmond, Virginia
VA Long Beach Healthcare System, Long Beach, California
Saarland University Hospital, Homburg/Saar, Germany
Henry Dunant Hospital, Athens, Greece
# Roche Laboratories Inc., Nutley, New Jersey
__ Roche Diagnostics, Pleasanton, California
University of North Carolina, Chapel Hill, North Carolina
ABSTRACT
Background & Aims: To maximize sustained virologic response (SVR) in patients with chronic hepatitis C virus (HCV) infection, treatment with pegylated interferon and ribavirin has been genotype-specific (1 vs non-1). We evaluated the effects of ribavirin and peginterferon alfa-2a dose reductions on SVR in patients infected with HCV genotype 1.
Methods: Data were pooled from 569 patients enrolled in 2 phase III trials of 48 weeks of treatment with peginterferon alfa-2a and ribavirin. All patients were evaluated for the effect of cumulative drug exposure on 4- and 12-week responses, and the 427 patients who completed treatment were evaluated for effect of drug exposure on SVR.
Results: Of patients who completed treatment, more had reductions (≦97% cumulative dose) of ribavirin than of peginterferon alfa-2a (43% vs 27%). Neither early virologic response nor SVR was affected adversely by ribavirin reductions when the cumulative ribavirin exposure was greater than 60%. The SVR was reduced significantly (P = .0006) in patients with less than the 60% cumulative ribavirin dose and was associated with prolonged periods of dose reduction, temporary interruptions, or premature cessation of ribavirin. Ribavirin dose reductions had minimal impact on SVR in patients who achieved rapid virologic response, defined as undetectable HCV RNA levels after 4 weeks, even when they received less than the 60% cumulative ribavirin dose. In contrast, SVR was reduced markedly in patients who had ribavirin dose reductions and did not achieve rapid virologic response.
Among the patients who completed 48 weeks of treatment, 76 had ribavirin dose reductions through week 12 for safety reasons, including both adverse events and laboratory abnormalities; of these 76 patients, 37 (49%) achieved SVR. SVR rates were higher for those with reductions as a result of safety reasons after week 12 (62 of 102, 61%) and those with ribavirin dose reductions for other reasons (124 of 190, 65%).
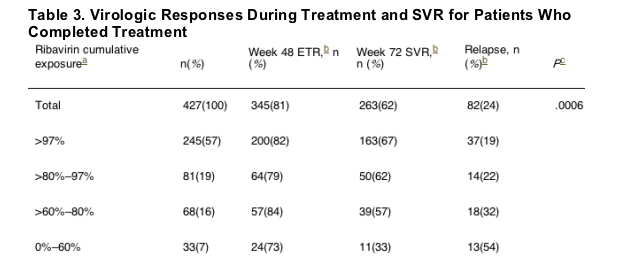
Table 3 shows that, as cumulative ribavirin exposure was reduced, there was a significant decrease in SVR (P = .0006): when RBV cumulative exposure was >97% 62% had SVR & 19% relapse rate; when RBV cumulative exposure was 80-97% SVR was 62% & 22% relapse rate; RBV cumulative exposure of 60-80% resulted in SVR of 57% & relapse rate of 32%; cumulative RBV exposure of 0-60% resulted in SVR of 33% & 54% relapse rate.
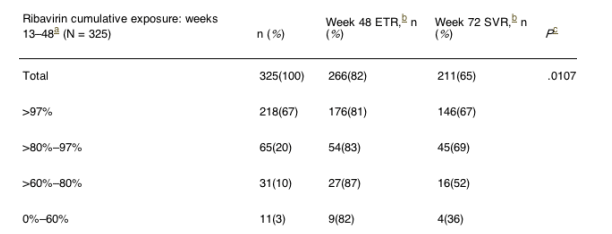
Table 4 shows that ribavirin dose reductions after week 12 for patients with earlier optimal exposure had a negative impact on SVR when the cumulative exposure during weeks 13-48 was 80% or less: RBV cumulative exposure, weeks 13-48 (n-325), when >97% SVR was 67%, when 80-97% cumulative RBV exposure SVR was 69%, when 60-80% cumulative RBV exposure SVR was 52%, and when 0-60% cumulative RBV exposure SVR was 36%.
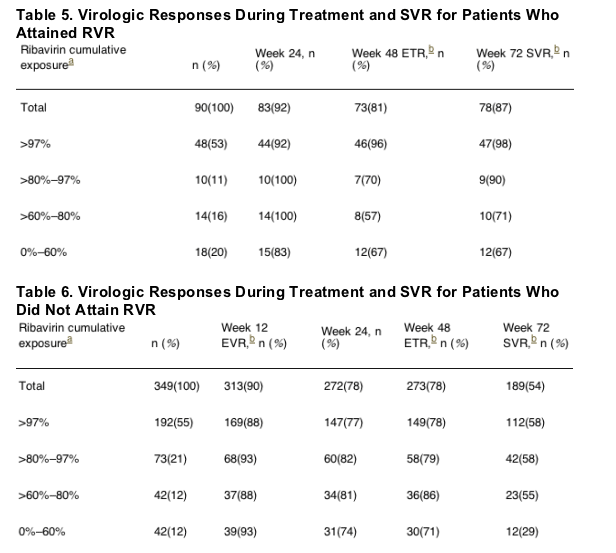
For patients who achieved an RVR: cumulative RBV exposure of >97% resulted in 98% SVR; 80-97% RBV cumulative exposure resulted in 90% SVR; 60-80% RBV cumulative exposure resulted in 71% SVR; 0-60% RBV cumukative exposure resulted in 67% SVR. See table 5 below.
Conclusions: Minor ribavirin dose reductions to manage adverse events do not appear to affect SVR adversely, unless cumulative exposure is less than 60%. Prospective studies, however, are required to establish the impact of ribavirin dose reduction on SVR.
Results
Of the 1121 patients in 1 study3 and 1284 in the second study,4 453 and 436, respectively, were randomized to treatment with peginterferon alfa-2a (180 μg/wk) plus ribavirin (1000 or 1200 mg/day) for 48 weeks and 298 and 271 patients, respectively, were infected with HCV genotype 1 and deemed eligible for this analysis. Of these 569 eligible patients, 427 (75%) completed 48 weeks of treatment, whereas 142 (25%) patients withdrew from treatment before 48 weeks.
Per protocol, the ribavirin dose was to be reduced from 1000 or 1200 mg/day to 600 mg/day if hemoglobin levels decreased to less than 10 g/dL in patients who did not have significant cardiovascular disease.
Frequency of Peginterferon Alfa-2a and Ribavirin Dose Reductions and Sustained Virologic Response
Of the 427 patients who completed 48 weeks of treatment with peginterferon alfa-2a and ribavirin, 182 (43%) had reductions or discontinuations of ribavirin at some point during treatment, making their total ribavirin exposure 97% or less. In contrast, only 114 of the 427 patients (27%) who completed peginterferon alfa-2a treatment received 97% or less of that drug. Table 1 shows the numbers of treatment completers in the various cumulative dose categories. Among the 313 patients who maintained full exposure to peginterferon alfa-2a, 120 (38%) had ribavirin dose reductions; in contrast, of 245 patients who maintained full exposure to ribavirin, only 52 (21%) had peginterferon alfa-2a dose reductions. Table 1 also shows the impact of the cumulative dose of peginterferon alfa-2a and ribavirin on SVR. Because the sample sizes for lower degrees of peginterferon alfa-2a exposure were too small, the analyses in this report focus on patients who completed treatment and had ribavirin dose reductions.
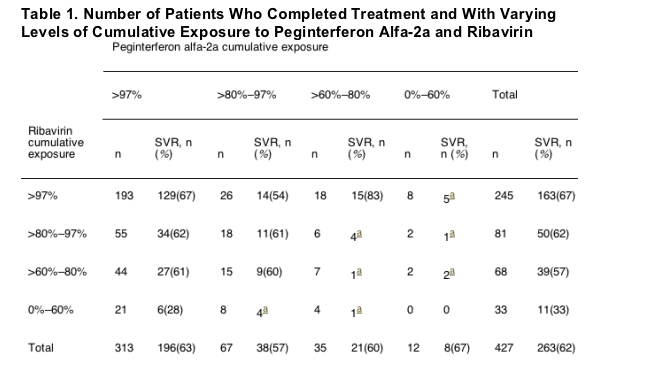
Reasons for Ribavirin Dose Reduction and Relationship to Sustained Virologic Response
The reasons for ribavirin dose reduction were identified for all patients before week 12 and for patients who completed treatment. Of the 545 patients who completed 12 weeks of treatment, 456 (84%) achieved EVR (Table 2). Among the patients who completed 48 weeks of treatment, 76 had ribavirin dose reductions through week 12 for safety reasons, including both adverse events and laboratory abnormalities; of these 76 patients, 37 (49%) achieved SVR. SVR rates were higher for those with reductions as a result of safety reasons after week 12 (62 of 102, 61%) and those with ribavirin dose reductions for other reasons (124 of 190, 65%). Anemia was the cause of ribavirin reductions for safety in 46 of 76 treatment completers before week 12 (61%), compared with 50 of 102 (49%) completers after week 12. Further analysis showed that most of the former also had low cumulative exposures to ribavirin; of these 76 patients, 60 (79%) had 80% or less, and 29 (38%) had 60% or less of their planned ribavirin exposure. Thus, low ribavirin exposure throughout the treatment period was associated with early dose reduction owing to safety reasons. In addition, about one third of all patients who had early dose reductions because of safety reasons discontinued treatment altogether. In contrast, among patients with dose reductions because of safety reasons after week 12, more than half were able to reach greater than 80% cumulative exposure.
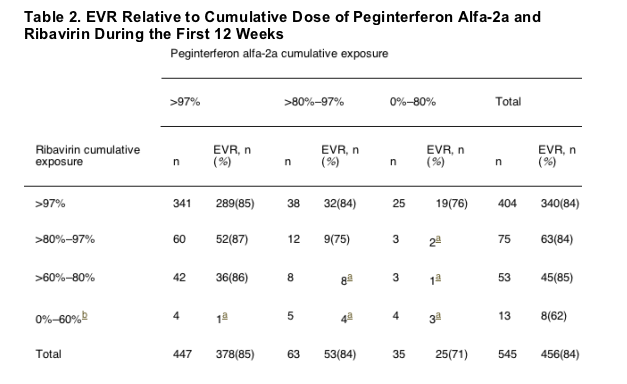
Relationship of Ribavirin Exposure to End-of-Treatment Response and Sustained Virologic Response
Suppression of HCV RNA for those patients in all categories who remained on treatment continued to be effective, as shown by the ETR rate, except for a somewhat lower ETR rate for patients with 60% or less ribavirin exposure. Table 3 shows that, as cumulative ribavirin exposure was reduced, there was a significant decrease in SVR (P = .0006). We also calculated the SVR rate for each group of patients who completed treatment while maintaining greater than 97% of their peginterferon alfa-2a dose. We found that SVR was attained by 11 of 33 (33%) patients who received 60% or less of the total ribavirin dose, compared with 252 of 394 (64%) patients who received greater than 60% of the total ribavirin dose (P < .0001). The patients with less than 60% of the cumulative ribavirin dose had either prolonged periods of dose reduction or interruption of therapy (21 of 33 patients) or had prematurely discontinued ribavirin (12 of 33 patients). The SVR rate was 30% (7 of 21) for the first group and 33% (4 of 12) for the second group. In addition, when we calculated the relapse rate relative to cumulative ribavirin exposure over the 48-week treatment period, we found that the relapse rate increased when the ribavirin exposure level decreased.
To address the issue of the impact of dose reductions after week 12, we evaluated patients who had greater than 97% ribavirin exposure up to week 12 and then a reduced dose during weeks 13-48. Table 4 shows that ribavirin dose reductions after week 12 for patients with earlier optimal exposure had a negative impact on SVR when the cumulative exposure during weeks 13-48 was 80% or less (P = .0372; odds ratio, 0.79).
Table 4. Virologic Responses According to Ribavirin Exposure During Weeks 13 to 48 for Patients Who Received More Than 97% of Their Ribavirin Exposure During Weeks 1-12 and Completed Treatment
Although a decrease in the cumulative ribavirin exposure levels appeared to have a direct negative effect on SVR, we were concerned that if those with lower exposure to ribavirin also had lower exposure to peginterferon then the interpretation would be confounded. Therefore, we determined the proportion of patients with more than 80% and 80% or less cumulative exposure to peginterferon alfa-2a (derived from Table 1). We found that patients with ribavirin reductions were not more likely to have peginterferon reductions because 87%-90% of patients in all ribavirin dose categories had more than 80% exposure to peginterferon alfa-2a. In addition, for patients with more than 80% peginterferon alfa-2a exposure, SVR rates at each ribavirin exposure level were similar to those of the entire group (65%, 62%, 61%, and 34% for decreasing levels).
Relationship Between Rapid Virologic Response and Ribavirin Dose Reductions During Weeks 5-48
We also addressed the impact of ribavirin dose reductions during weeks 5-48 on SVR in patients with and without RVR (Table 5, Table 6). We found that patients who achieved RVR were more likely to achieve SVR, both in general and for each cumulative ribavirin dose. For patients who achieved RVR, very high SVR rates were observed in the 70% range, even with ribavirin dose reductions to 80% or less (Table 5). In contrast, in patients who did not achieve RVR, ribavirin dose reductions after week 4 had an impact on overall SVR rates in all exposure categories when compared with those who achieved RVR (Table 6). Furthermore, patients who achieved RVR were less likely to relapse (Table 5) than patients who did not achieve RVR (Table 6).
Relationship Between Rapid Virologic Response and Ribavirin Dose Reductions During Weeks 5-48
We also addressed the impact of ribavirin dose reductions during weeks 5-48 on SVR in patients with and without RVR (Table 5, Table 6). We found that patients who achieved RVR were more likely to achieve SVR, both in general and for each cumulative ribavirin dose. For patients who achieved RVR, very high SVR rates were observed in the 70% range, even with ribavirin dose reductions to 80% or less (Table 5). In contrast, in patients who did not achieve RVR, ribavirin dose reductions after week 4 had an impact on overall SVR rates in all exposure categories when compared with those who achieved RVR (Table 6). Furthermore, patients who achieved RVR were less likely to relapse (Table 5) than patients who did not achieve RVR (Table 6).
Relationship Between Ribavirin Discontinuations and Sustained Virologic Response
In the analyses shown earlier, we compared treatment response with the cumulative dose of ribavirin. Because discontinuation of ribavirin was allowed as long as peginterferon alfa-2a treatment was maintained, we determined the number of patients with ribavirin discontinuations in the various exposure categories. None of the patients who completed treatment and had more than 80% exposure to ribavirin discontinued ribavirin therapy. Of the 33 patients who had ≦60% ribavirin exposure, 12 discontinued ribavirin whereas 21 did not. SVR was achieved by 33% of each of these subgroups (data not shown). This observation suggests that these 2 subgroups had a lower but equal probability of achieving SVR regardless of whether the ≦60% ribavirin exposure was owing to discontinuation or dose reduction.
Discussion
In this report we present a detailed analysis on the impact of ribavirin dose reductions on SVR rates in patients infected with HCV genotype 1 and naive to treatment who were treated with the combination of peginterferon alfa-2a plus ribavirin in 2 large randomized clinical trials.3, 4 We focused primarily on ribavirin dose reduction in this analysis because nearly half of the patients (43%) who remained on treatment for 48 weeks experienced ribavirin dose reductions, whereas reductions in peginterferon alfa-2a dose were less common (27%) in these patients. In addition, the exposure to peginterferon alfa-2a was roughly equivalent among the groups regardless of their exposure to ribavirin, indicating that peginterferon alfa-2a reductions were not more common among those with reduced ribavirin exposure and were not responsible for our findings.
Neither EVR nor SVR was affected adversely by mild-moderate reductions in ribavirin dose, but SVR rates were reduced in patients who received less than 60% of their cumulative ribavirin dose over the 48 weeks. Patients who received less than 60% of their cumulative ribavirin had prolonged periods with significant dose reduction, periods of temporary interruption of treatment, or complete cessation of ribavirin. Only 13 patients received less than 60% of their ribavirin dose during the first 12 weeks, a number too small to allow evaluation of the effects of significant ribavirin dose reductions on EVR.
Improvements in overall SVR among patients who had greater exposure to ribavirin appeared to be owing mainly to lower relapse rates. Thus, for those remaining on treatment, viral suppression continued to be effective. The higher relapse rates for patients with 60% or less ribavirin exposure were similar to rates observed for patients who did not clear virus early and required a longer period of treatment to achieve SVR.12, 13 RVR has been suggested as the strongest positive predictor of SVR, with patients achieving SVR thought to require a shorter duration of therapy than patients who do not eliminate HCV within the first month.14 This concept is consistent with the results of a large randomized trial that investigated the optimal time and duration of treatment according to HCV genotype.4 Patients with a shorter duration of treatment, even with the standard dose of ribavirin, had higher relapse rates. Thus, as a corollary, patients with low exposure to ribavirin during the 48-week treatment period may benefit from treatment beyond 48 weeks, with a reduced chance of relapse.
Although ribavirin has only a modest transient effect on HCV clearance in the absence of interferon,15, 16 it greatly enhances SVR rates when given in combination with interferon alfa.17, 18, 19 Ribavirin significantly accelerates the second/third phase of HCV clearance in patients treated with peginterferon, and treatment with peginterferon and ribavirin enhances the rates of EVR, ETR, and SVR relative to that with peginterferon alone.3, 11
In conclusion, the impact of ribavirin dose reduction on SVR rate may be minimal if patients complete treatment and maintain greater than 60% of their planned ribavirin dose. Reduced SVR rates were observed in patients who completed treatment, but with decreased exposure to ribavirin, in clinical trials with peginterferon alfa-2a and ribavirin. The data suggest that reduced ribavirin exposure may be correlated with increased rates of relapse. Although viral suppression while on treatment is effective, even with moderate ribavirin dose reductions, maintaining ribavirin and peginterferon alfa-2a dosing is essential to achieving SVR.
Background
Combination therapy with pegylated interferon plus ribavirin has become the standard of care in the treatment of patients with chronic hepatitis C virus (HCV) infection.1, 2, 3 For patients infected with genotypes 2 and 3, treatment for 24 weeks with 180 μg/wk peginterferon alfa-2a plus 800 mg/day ribavirin results in a sustained virologic response (SVR) in about 80% of patients. Higher doses of ribavirin and more prolonged therapy have not been shown to improve responses in these patients. For patients infected with HCV genotype 1, however, a higher dosage of ribavirin, 1000 to 1200 mg/day, and a treatment duration of at least 48 weeks are necessary to achieve an SVR rate of about 50%.4 Thus, patients infected with HCV genotype 1 are considered more difficult to treat, and more intensive treatment regimens have been suggested to maximize virologic response.5
For most therapeutic regimens, treatment success is correlated highly with drug exposure. Drug exposure depends on the pharmacokinetic properties of a drug and the ability of a patient to adhere to the drug regimen, as well as the amount of drug administered over a specific period of time. Although the relationship between exposure to antiretroviral drugs and treatment success has been studied in patients infected with the human immunodeficiency virus,6, 7 this relationship is less well understood for patients with HCV infection. There is some evidence that dose reductions negatively affect SVR rate in patients with genotype 1 HCV infection.8 Ribavirin dose reductions during the first 12 weeks of treatment were associated with a decreased rate of early virologic response (EVR), defined as undetectable or at least a 2-log reduction in HCV RNA level after 12 weeks of therapy.9 There is limited information, however, on the effects of ribavirin and/or peginterferon dose reductions later in therapy. In patients who were nonresponders to previous treatment with conventional (nonpegylated) interferon, with or without ribavirin, and who subsequently were treated with peginterferon alfa-2a plus ribavirin, ribavirin dose reductions during weeks 1-20 were associated with a reduction in SVR rate, from 21% to 11%. In contrast, reductions in ribavirin dose after week 20, or peginterferon alfa-2a dose reductions throughout the 48 weeks of treatment, had no effect on SVR rate.10 Ribavirin has been shown to increase the initial viral kinetic response to interferon, thus potentially improving EVR and overall efficacy of the treatment for hepatitis C.11
The hematologic toxicities of ribavirin and the observation that ribavirin dose reductions impair response to combination therapy have led to the widespread use of hematologic growth factors to treat ribavirin-associated anemia. Administration of these expensive therapies is thought to avoid ribavirin dose reductions, but there is no evidence that these growth factors have resulted in improvements in the overall response to treatment. The recommendation for the use of growth factors, however, depends in large part on the degree to which SVR is compromised by ribavirin dose reductions and the degree to which this reduced response can be reversed by growth factor administration. In addition, the timing of ribavirin dose reductions may affect SVR, thus influencing the timing of growth factor administration. To answer these questions, we retrospectively analyzed responses in the patients infected with genotype 1 who were included in registration trials of peginterferon alfa-2a plus ribavirin.3, 4 The objective of this analysis was to determine the relationship between cumulative exposure to peginterferon alfa-2a and ribavirin, the timing of dose reductions, and SVR in patients infected with HCV genotype 1.
Patients and Methods
Patients
This was a retrospective analysis of drug exposure in patients infected with HCV genotype 1 from the phase 3 multinational clinical trials of peginterferon alfa-2a plus ribavirin.3, 4 The study entry criteria have been well described.3, 4 A total of 569 patients infected with HCV genotype 1 were randomized to treatment for 48 weeks with peginterferon alfa-2a (180 μg/week) plus ribavirin (1000 mg/day for patients weighing ≦75 kg, or 1200 mg/day for patients weighing >75 kg) and followed-up for an additional 24 weeks after termination of treatment. Of the 569 randomized patients, 427 (75%) completed 48 weeks of treatment and follow-up evaluation. Patients were discontinued from the study if they remained positive for HCV RNA at week 24. Discontinuation of peginterferon only (ie, ribavirin monotherapy) was not allowed.
Dose Modification
Patients who experienced difficulty tolerating the dose of peginterferon alfa-2a were allowed dose reductions in decrements of 25% and 50% of the assigned dose. When the absolute neutrophil count decreased to less than 1000 cells/mm3, the dose was reduced to 135 μg; when the absolute neutrophil count was less than 750 cells/mm3, the dose was reduced to 90 μg; and when the absolute neutrophil count was less than 500 cells/mm3, the dose was reduced to 45 μg. If the absolute neutrophil count decreased to less than 250 cells/mm3, peginterferon alfa-2a was withheld/discontinued.
Per protocol, the ribavirin dose was to be reduced from 1000 or 1200 mg/day to 600 mg/day if hemoglobin levels decreased to less than 10 g/dL in patients who did not have significant cardiovascular disease. Similar reductions in the ribavirin dose were made in patients with stable cardiovascular disease who experienced a decrease in hemoglobin level of more than 2 g/dL during any 4 weeks of treatment. Ribavirin was to be discontinued if hemoglobin levels decreased to less than 12 g/dL in patients with significant cardiovascular disease or less than 8.5 g/dL in patients without cardiovascular disease despite 4 weeks on a reduced dose. The use of erythropoietin and granulocyte-stimulating growth factors was prohibited.
Analytic Methods
Definitions
The HCV RNA level was measured using the CoBAS Amplicor HCV Test, version 2.0 (lower limit of detection 50 IU/mL; Roche Diagnostics, Branchburg, NJ). The rapid virologic response (RVR) was defined as undetectable serum HCV RNA at 4 weeks. EVR was defined as at least a 2-log10 decrease in HCV RNA or undetectable serum HCV RNA at 12 weeks. End-of-treatment response (ETR) was defined as undetectable HCV RNA at week 48, and SVR was defined as undetectable serum HCV RNA at week 72, or 24 weeks after treatment discontinuation. The relapse rate was defined as follows: (%ETR - %SVR)/%ETR.
Drug exposure
Drug dispensing logs were kept for all patients, including the quantity of drug dispensed to and returned by the patient. Cumulative drug exposure was defined as the total dose of each drug received by a patient, less the amount returned, expressed as a percentage of the planned total dose. There were 4 exposure categories for each of the 2 drugs: greater than 97%, which allowed missing 1 dose of peginterferon alfa-2a or 1 week of ribavirin; greater than 80% to 97%; greater than 60% to 80%; and 60% or less. Cumulative drug exposure was evaluated for the full 48 weeks of treatment and during weeks 0-12. For exposures before week 12 all patients were evaluated, whereas for exposures during weeks 13-48 only the 427 patients who completed treatment were considered. In addition, patients were divided into those who did and did not achieve RVR, and the effects of ribavirin dose reductions during weeks 5-48 were evaluated.
Statistical analysis
Logistic regression was used to analyze the relationship between the cumulative ribavirin level and SVR. The Mantel-Haenszel χ2 test was used to test the difference between the 2 proportions. The Cochran-Armitage linear trend exact test also was used to determine the linear trend between the categoric ribavirin cumulative dose and SVR.
|
|
| |
| |
|
|
|