| |
Combination Therapy With Telaprevir and Pegylated Interferon Suppresses Both Wild-Type and Resistant Hepatitis C Virus
|
| |
| |
Gastroenterology Jan 2007
New clinical research presented at the 57th Annual Meeting of the American Association for the Study of Liver Diseases in October suggests that both wild-type hepatitis C virus (HCV) and resistant variants were suppressed in patients when pegylated interferon (peginterferon ƒ¿-2a; peg-IFN) was added to telaprevir, an investigational HCV protease inhibitor from Vertex Pharmaceuticals (Cambridge, MA).
The study analyzed viral sequences isolated from patients receiving telaprevir (VX-950) as a single agent or in combination with peg-IFN in a Phase 1b 14-day clinical study. In this study, viral variants were suppressed when peg-IFN was combined with telaprevir, or when peg-IFN and RBV were administered subsequent to telaprevir dosing (Figure 1).
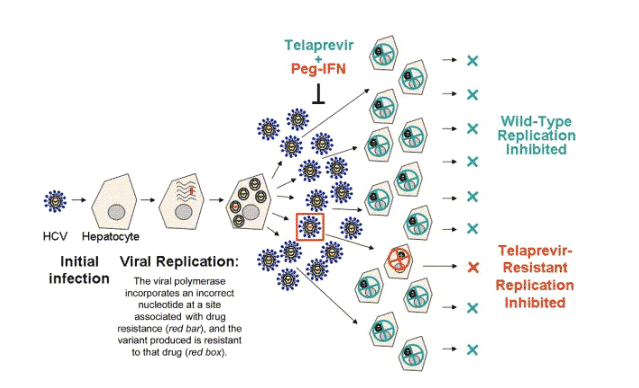
Figure 1. Telaprevir + Peg-IFN prevent replication of wild-type and resistant virus. After initial infection of a hepatocyte, HCV begins to replicate and at some frequency the viral polymerase will incorporate the wrong nucleotide in the RNA, generating viral variants. When the mutation occurs at a site that is associated with drug resistance, shown here in red, the variant released will be resistant to that drug. With high levels of replication in an untreated patient, point mutations at each position in the genome arise at least once every day, so resistant virus is constantly being generated. Therefore, most drug-resistant variants likely pre-exist within the viral quasispecies and are selected from within this heterogeneous environment upon application of selective drug pressure. Treatment with a specifically targeted antiviral therapy for HCV (STAT-C), telaprevir, profoundly inhibits replication of wild-type virus and resistant virus is relatively more fit and can be selected in the presence of the drug. However, with the addition of Peg-IFN to the telaprevir treatment, replication of both wild-type and resistant replication is inhibited.
"In the trial, 8 patients were treated with telaprevir alone and 8 with a combination telaprevir and Interferon. All of the patients had an initial decline in viral load," said Dr Tara Kieffer, research scientist with Vertex. "And in the group that received telaprevir and interferon, all patients continued to respond. We saw no clinical breakthrough in any of those patients, even though we did detect resistant variants in some patients."
Dr Kieffer reported that resistant viral variants were isolated from 6 of 8 patients who had detectable HCV RNA while receiving telaprevir as a single agent over a period of 14 days. Subsequently, clinical investigators reported that all patients who received follow-on therapy with peg-IFN and RBV had undetectable HCV RNA at 24 weeks. In a second arm of the study, resistant viral variants were isolated from 2 of 8 patients who received a combination of telaprevir and peg-IFN for 14 days. Both patients subsequently had undetectable HCV RNA at weeks 12 and 24 of follow-on therapy, Dr Kieffer adds. "Previously we have shown that telaprevir resistant viral variants were sensitive to interferon in vitro. This trial has shown that you can detect these variants in vivo, in patients. But in the eight patients that received interferon along with telaprevir, the viral load does continue to decline," Dr Kieffer said. She notes that further clinical studies in Germany are continuing for up to 48 weeks with the patients in the Phase 1b trial. Although viral suppression on interferon and telaprevir is impressive, the ultimate sustained response off of therapy is what matters and the results are eagerly awaited.
|
|
| |
| |
|
|
|